Chemicals like 4-Morpholinecarbaldehyde didn’t just show up on lab shelves overnight. Decades of research led up to its current spot in science and industry. Early experiments with morpholine go back to the golden age of organic chemistry, around the time researchers started linking nitrogen-containing rings to practical applications. Once chemists found a way to tweak morpholine’s structure to tack on an aldehyde at the 4-position, 4-Morpholinecarbaldehyde was born. Its creation marked a big shift for synthetic routes that previously relied on less stable, less predictable intermediates. Over time, improved purification, reaction yields, and a hunger for new building blocks in pharma and agrochemical labs cemented it as a useful specialty chemical.
4-Morpholinecarbaldehyde serves as much more than a line on a chemical catalog. Its straightforward aldehyde group sits on a tough, ring-shaped backbone. You don’t see it by itself in the wild; most action comes in research, custom synthesis, or as a stepping stone in more complex molecule builds. Bench chemists like its small size and its knack for clean reactions. With its shape and balance of hydrophilic and hydrophobic traits, this compound neatly fits into screening libraries, pharmaceutical intermediates, and even fine-tuning performance in specialty coatings.
Delving into the bottle, you’ll find a colorless to pale-yellow liquid or sometimes a crystalline solid, depending on storage and grade. Its molecular weight hangs at 129.15 g/mol, a manageable figure for most synthetic work. The boiling point hovers around 240°C, though it may decompose before reaching that temperature, so gentle heating counts. It dissolves well in polar solvents—think methanol, ethanol, and water—making it versatile during reactions or downstream isolation. Its melting point, set around 18-20°C, means it can flow or solidify, depending on the room conditions. Like other aldehydes, 4-Morpholinecarbaldehyde proves reactive with nucleophiles and works as a decent electrophile under mild conditions.
Labels on commercial bottles of 4-Morpholinecarbaldehyde don’t just carry a name. Purity often goes above 97%, with water content and color index closely tracked. Impurity profiles get reported, especially if the destination is pharmaceutical research or regulatory filings. You might spot “for research use only” if you order some. That comes with extra paperwork in certain regions. Handling instructions, hazard codes, and physical constants appear right on the label or technical data sheet to keep users ahead of safety issues.
No industrial chemist enjoys fussing over uncertain routes, so most stick to oxidation of 4-methylmorpholine through methods like Swern, Dess-Martin, or using manganese dioxide. The basics stay the same: Start with morpholine, work up some methylation at the fourth position, and finally coax a careful oxidation to drop in the aldehyde. Some labs branch into catalytic routes, but most commercial outfits rely on established methods, balancing cost, scalability, and purity. Efficient routes pay off, especially if you need sizable batches for follow-up reactions or pilot plant tests.
What makes 4-Morpholinecarbaldehyde extra valuable is where it leads the next reaction step. The aldehyde brings out the familiar moves: condensation, reductive amination, nucleophilic addition, and Wittig olefination. Its ring holds up to surprisingly harsh conditions, so hard-nosed reagents like Grignards, organolithiums, or even enzymatic reduction can target the carbonyl group without shredding the morpholine backbone. Chemists use it to make amines, imines, and even push into heterocyclic frameworks that would feel out of reach with more fragile starting materials.
Names don’t always stay simple in chemical catalogs. 4-Morpholinecarbaldehyde might show up as 4-Formylmorpholine, 4-Aldehydomorpholine, or N-Oxydiethyleneformamide. Each synonym still marks the same aldehyde hanging off the fourth position. Some suppliers tag on “for synthesis” or use non-English terms, like “Morpholin-4-karbaldehyd.” Any search through chemical inventories should try at least a few of these options to catch every available grade and supplier.
Handling chemicals like this one isn’t just about gloves and goggles. 4-Morpholinecarbaldehyde brings the typical hazards you’d expect from both aldehydes and nitrogen-based heterocycles. Skin or eye contact triggers irritation, and inhalation could pose respiratory risks. Regulatory guidance from OSHA or REACH lists ventilation, splash protection, and fume hood use as critical precautions. Waste goes out according to aldehyde disposal standards—treated oxidatively or incinerated with other organic waste. Chemical hygiene plans in academic or industrial settings spell out procedures for handling spills, controlling vapors, and emergency eyewash use. Practical experience shows that respecting these steps avoids lost time and protects team health.
In the last ten years, 4-Morpholinecarbaldehyde proved itself far beyond a niche research tool. Its main stretch lies in medicinal chemistry—serving as both a core and as a reactive handle for building lead candidates. Beyond pharma, agrochemical researchers lean on it to devise new pesticides or fungicides built to last in harsh field environments. Oddly enough, it even crops up in the electronics sector, guiding molecular templates for functional coatings or as a specialty cross-linker. Small-scale custom synthesis shops often turn to it for pilot batches requested by universities or startups testing new bioactive molecules.
R&D using 4-Morpholinecarbaldehyde has ramped up in labs targeting combinatorial chemistry or fragment-based drug discovery. Its aldehyde lets chemists “click” together molecule libraries using reliable reactions. My own work in a university lab counted on reagents like this one for late-stage functionalization, where selectivity and reliability matter. In industry, automation tools now allow researchers to build dozens if not hundreds of derivatives in parallel, using the morpholine scaffold as a launchpad to discover new properties. New preparative routes roll out every few years, pushing for greener, cheaper, and faster approaches to get more out of every reaction flask.
No new chemical earns a role in pharmaceuticals or fine chemicals unless toxicologists dig into its potential health risks. Studies point out that 4-Morpholinecarbaldehyde holds moderate toxicity, with oral LD50 values in the low to mid hundreds of mg/kg in rodents. Standard in vitro studies show potential for tissue irritation, largely pinned on the aldehyde group’s reactivity. Mutagenicity screens seem less worrying, but close monitoring continues, especially as researchers seek clearance for use in the pharmaceutical pipeline. Proof from animal studies gets weighed against structural alerts common to aldehydes, and anyone using it in a regulated lab must record exposures and handle spills promptly.
The horizon looks busy for 4-Morpholinecarbaldehyde. Generative AI tools now pitch novel reaction pathways using molecular building blocks just like it, making it easier than ever for chemists to morph ideas into real compounds. Rising demand for specialty pharmaceuticals—especially ones shaped by flexible, nitrogenous scaffolds—means more companies will place orders for derivatives and analogs. Ongoing work in “green chemistry” finds new ways to rework preparation without tricky oxidants or heavy metals, dropping costs and boosting sustainability. Research on targeting new disease pathways makes the morpholine-aldehyde combination a hot commodity for exploring bioactivity in uncharted therapeutic classes. As innovation cycles grow shorter, compounds like this one offer a launchpad for the next big molecule in healthcare, agriculture, and electronic materials.
Chemistry often hides some of its most interesting characters away from the spotlight. 4-Morpholinecarbaldehyde, with its mouthful of a name, lives in this category. I’ve encountered this compound most often during conversations with people working in labs focused on pharmaceutical research. It’s not a commonly discussed chemical outside technical circles, but the roles it plays end up touching many lives.
On the surface, 4-Morpholinecarbaldehyde acts as a functional aldehyde for making more complex molecules. Researchers use it to build bigger drugs, test reaction pathways, and pin down unique structures that seem promising for new medicines. It steps in during medicinal chemistry when scientists need to fine-tune how a molecule behaves. The morpholine ring helps add water-solubility or control the electronic “push and pull” of the molecules it attaches to, allowing chemists to shape drugs that dissolve better or move more easily through the body.
One of the more interesting facts I learned while diving into journal articles was about its role in tweaking antibiotics and antifungals. By attaching new functional groups to the morpholine scaffold, researchers have sometimes managed to sidestep resistance—the ever-present threat where germs outsmart current drugs. It gives scientists a solid foundation to experiment on without starting from scratch each time.
Outside of drug discovery, 4-Morpholinecarbaldehyde pops up as a reactant in making other fine chemicals. It assists with making specialty dyes and pigments that show up in everything from medical diagnostic stains to some industrial inks. I recall speaking with a chemist who used derivatives for analytical chemistry. Its ability to form stable linkages with biomolecules made it valuable for labeling or tracking compounds in complex mixtures, work that can reveal how fast an enzyme chews through sugar or how proteins interact in living cells.
It also finds work alongside catalysts as a ligand, helping metals bind in just the right fashion for important reactions. Its morpholine ring, with its nitrogen and oxygen, offers a flexible grip for researchers trying to guide metals through tricky transformations.
Even if it wears many hats in laboratories, this compound raises real issues. Like most aldehydes, it comes with risks. Exposure through skin or breathing has consequences, from irritation to long-term effects if handled carelessly. Many chemists I know respect its reactive bite and keep strict safety routines—good gloves, proper ventilation, and solid waste management. Those practices matter, not just for safety, but for the environment. Disposal needs attention. It can’t just be poured down the drain. Every chemist who’s ever trained new students will recall reinforcing those basic lessons.
With environmental concerns growing louder every year, paying attention to chemicals like this means not only handling them safely but considering greener alternatives where possible. Some labs now use bio-based solvents instead of harsher ones in reactions that use 4-Morpholinecarbaldehyde. There’s room for improvement, and actively searching for safer processes should never feel optional.
In a world where new diseases emerge and technical needs evolve, 4-Morpholinecarbaldehyde remains a reliable tool for building, experimenting, and discovering. Its best value comes from those willing to match deep knowledge with careful practice—and a mindset of looking ahead, always weighing the benefit against the cost to health and planet.
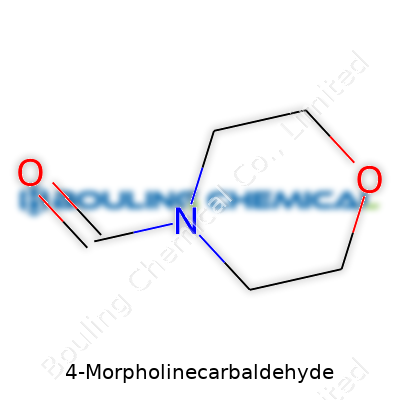
4-Morpholinecarbaldehyde gets attention in research labs and chemical manufacturing because it shows up in the development of pharmaceuticals and specialty chemicals. Folks often overlook the basics, but the molecular formula matters a lot. 4-Morpholinecarbaldehyde contains a morpholine ring—a structure with four carbons, one oxygen, and one nitrogen—attached to an aldehyde group.
The molecular formula of 4-Morpholinecarbaldehyde is C5H9NO2. Let’s look at the anatomy of this compound. The morpholine ring shapes up with four carbon atoms in the backbone, connected by one oxygen and one nitrogen. The “4” in the name marks the position where the aldehyde group attaches—right on the fourth carbon atom in the ring.
Here’s how the count works out. Start with the four carbons forming the ring, then add one more carbon from the aldehyde group. The ring already carries one oxygen and one nitrogen, and the aldehyde brings in an extra oxygen. Add up the hydrogens around the ring and at the end groups, and that delivers nine hydrogens. So, the sum is five carbons, nine hydrogens, one nitrogen, two oxygens.
Getting the formula right isn’t just for paperwork. A formula shows what you’re working with at the atomic level. For researchers in medicinal chemistry, knowing the correct ratio ensures safe handling, accurate reactions, and reliable results. If someone mistakes one atom, it can mean disaster—for instance, a toxic byproduct or a reaction that fails. No chemist takes that gamble.
Pharmaceutical teams use 4-Morpholinecarbaldehyde as a building block. In small-molecule drug discovery, chemists tweak formulas to fine-tune how a drug interacts with the body. A clear formula also helps with regulatory work. Governments expect full transparency about chemicals moving in and out of markets. Getting audited with errors on the formula brings penalties, sometimes even bans.
Outside of drugs, specialty chemicals companies rely on this molecule in dyes and flavors. Each application needs the right version, so an accurate formula keeps production lines running without setbacks.
Supply chains and safety standards continue to challenge the industry. One big problem: mislabeled chemicals. It happens when someone gets lazy with labeling or assumes two formulas represent the same compound. They don’t. I’ve seen what the fallout looks like—confused lab workers, wasted resources, legal investigations. The solution always starts at the ground level: teach and review the chemistry basics, and keep tight inventory logs.
Labs also use digital tracking. Modern inventory software flags mismatches between what’s ordered, delivered, and logged in the database. Companies invest in these tools to cut down on human error and to automate checks. Regular training for chemists, including hands-on sessions in structure-checking, reduces mistakes further. The community around chemical handling needs to stay sharp as new regulations come out.
A reliable formula lets chemists all over the world communicate clearly. When teams get the formula for 4-Morpholinecarbaldehyde right, the chemical pipeline moves smoothly from lab notebooks into real-world solutions. Precision at the molecular level leads to meaningful science and safer products.
4-Morpholinecarbaldehyde, with its role in pharmaceutical and chemical synthesis, gets attention because of its reactive aldehyde group and the morpholine ring. Many folks in labs already know the basics of storing chemicals, but this compound reminds us why careful storage can mean the difference between smooth research and ruined samples—or worse, safety incidents.
This compound tends to absorb moisture from the air (hygroscopic). I’ve worked with aldehydes before, and letting even a small amount of moisture sneak in can spoil the sample’s purity. So, a tightly sealed glass container remains essential. Not just any old cap, either—a well-fitted stopper keeps the air out and helps maintain integrity. Even at home, the wrong lid lets your leftovers spoil; at the bench, the stakes climb quickly.
Aldehydes oxidize and degrade, especially above room temperature. I kept another sensitive aldehyde at ambient conditions, and within days, it yellowed and gave off a faint odor—a classic warning sign. Storing 4-Morpholinecarbaldehyde in a cool, dry place minimizes unwanted changes. For longer storage, around 2–8°C in a refrigerator works best. Never freeze unless you have a stability sheet that specifically recommends it. Substances often expand and contract, containers crack, and upon thawing, you could find a mess or even a dangerous byproduct.
Direct sunlight speeds up degradation. Even room lighting can trigger reactions in some aldehydes. While many labs keep chemicals in clear glass, an amber vial gives extra protection against light, and a little caution goes far. Cross-contamination strikes more often than folks like to admit. Once, in a rush, I used a spatula that wasn’t dry and ended up with a gummy, useless compound. Always use dry, clean equipment and never dip anything into the original container that has touched another chemical or even water.
A clear, complete label saves headaches down the line: include the compound name, the date you received it, and relevant hazard information. I’ve lost count of the times a poorly labeled bottle became a guessing game after sitting on a shared shelf. Inventory should stay up-to-date—expired chemicals invite risks. SDS (Safety Data Sheet) access remains non-negotiable. Reading through one before handling the substance can reveal hidden hazards. Aldehydes can irritate the eyes and lungs, and exposure sometimes happens through carelessness over time. Gloves, goggles, and a well-ventilated space aren’t negotiable for aldehyde handling, no matter how experienced someone thinks they are.
Disposal is rarely glamorous, but it matters. 4-Morpholinecarbaldehyde, like other aldehydes, demands thoughtful disposal through an approved chemical waste stream. Pouring even small amounts down the drain threatens water safety. Working with environmental health and safety staff in your facility pays off—they know the ins and outs of compliant, safe practices. No one loves bureaucracy, but public health depends on it in this case.
Many accidents in labs trace back to simple neglect of storage basics. Following these steps protects not only those handling 4-Morpholinecarbaldehyde but also everyone who shares the workspace. Proper conditions cut down waste, unnecessary expenses, and potential hazards. Safe storage protects scientific progress—and people.
4-Morpholinecarbaldehyde, known in some labs as 4-formylmorpholine, doesn’t show up on every industrial or academic shelf. Still, those who handle specialty chemicals might recognize it from synthetic routes or in the making of pharmaceuticals. I’ve witnessed chemists take a cautious approach even with unfamiliar substances, and with good reason: each compound comes with its own set of risks, and some aren’t as well-documented as we'd like.
For 4-Morpholinecarbaldehyde, the immediate concern starts with its chemical nature. This compound has an aldehyde group, notorious for causing irritation to skin, eyes, and lungs. Take formaldehyde — a well-known aldehyde. A few drops in the air can quickly sting the eyes and nose, so people wear gloves and work in fume hoods as a rule, not just as a recommendation.
Reliable safety data sheets, like those provided by Sigma-Aldrich, usually point out a handful of consistent hazards: irritation, potential toxicity if swallowed or inhaled, and possible sensitization. Having spent time in busy university labs, I saw what even a moment of unsafe handling could do. I remember a colleague accidentally splashing a related compound on their skin, ignoring the quick wash, and developing redness within minutes. That drives the point home: just because a chemical isn’t well-known doesn’t mean it’s harmless.
It doesn’t help that 4-Morpholinecarbaldehyde isn’t as widely studied as acetone or methanol. Most of the toxicity assessments come from basic animal studies or in-vitro predictions. That leaves us in a gray area, and I have learned not to gamble with gray areas. In places with strict workplace safety protocols — think pharmaceutical manufacturing or research institutions — anything lacking detailed toxicology gets the same respect as a known irritant.
Personal protective equipment separates safe labs from disaster scenes. Anyone working with 4-Morpholinecarbaldehyde should use nitrile gloves, lab coats, and splash goggles. My experience shows that chemical-resistant gloves help, since aldehydes can slip through latex. A certified chemical fume hood does more than containing smells; it forms a barrier against invisible vapors that corrode airways with silent efficiency.
Spills get cleaned up with absorbent pads designed for organics, and waste goes in labeled containers for hazardous materials pickup. This isn’t about rules — it’s about people. Years in shared lab environments made me respect the safety culture that keeps everyone going home in one piece. Telling new students that “you’ll get used to it” misses the point; preparation outperforms luck every time.
Training sets the foundation. Newcomers should get more than a mandatory video. Real conversations about past incidents and practical demonstrations matter. In my own labs, the difference between a written policy and a hands-on lesson was night and day. This isn’t about not trusting people; it’s about not trusting luck.
Accessible information makes a difference. Labeling containers clearly and keeping updated safety data sheets within arm’s reach saves time during emergencies. Labs benefit from regular audits and discussions after any close call. Encouraging questions instead of treating them as interruptions fosters a safer environment.
4-Morpholinecarbaldehyde doesn’t demand panic, but it does deserve the same respect given to any chemical with unknowns. Caution born out of experience and awareness shields against complacency — and, ultimately, keeps the work safe and productive.
Every bottle and drum in a lab holds more than a liquid or powder. It carries a story about getting the right results, avoiding danger, and improving trust across the chemical supply chain. If you jump onto a chemical catalog, you’ll see numbers beside chemical names everywhere. These identifiers hook directly into E-E-A-T principles: they cut out any guesswork, push for trust, and keep everything transparent. For 4-Morpholinecarbaldehyde, that number is 4394-85-8. Such a small label, but without it, people make expensive or dangerous mistakes. A wrong molecule ends up in a beaker, or data gets skewed in a research paper.
A Chemical Abstracts Service (CAS) number acts like a passport for molecules. I’ve worked in research spaces where two compounds sounded nearly identical, but their CAS numbers told completely different stories. Chemists, warehouse managers, logistics teams, even customs officials rely on these codes. In the case of 4-Morpholinecarbaldehyde, its structure—an aldehyde hanging off a morpholine ring—doesn’t just need a fancy diagram in a textbook. Its CAS number connects lab ordering systems, safety guidelines, and tracking for environmental regulations. Without it, researchers scramble, and supply chain safety falls apart. One slip in that chain, and somebody risks exposure, failed results, or loss of funding.
Picture this: a laboratory technician orders what they thought was 4-Morpholinecarbaldehyde for a pharmaceutical project. The container arrives, but the name is a letter or two off. Maybe the supplier meant well, using a trade name or a similar description. If the CAS number wasn’t checked closely, the experiment could derail. In 2014, a report from the American Chemical Society highlighted a case where an incorrect compound delivered under an ambiguous name ended up contaminating a clinical trial. That chain of events cost the company millions and delayed patient access to new medication by years.
Using a proper identifier like CAS 4394-85-8 bridges language gaps and corporate jargon. Suppliers serve buyers across continents with different alphabets, so universal numbers smooth communication and keep projects on track. When chemists or engineers teach newcomers to double-check CAS numbers, they’re focusing on more than lab efficiency—they’re reducing waste and limiting hazardous exposure. My first chemistry mentor drilled this into me by insisting we log both the formula and the CAS number for every reagent in the lab notebook. That record-keeping habit saved time during audits and prevented countless headaches with suppliers.
With the global trade in chemicals expanding, regulators use CAS numbers to flag dangerous substances crossing borders. Enforcement agents don't memorize structures or local synonyms—they look for those numbers. By tracing a single molecule, they stop unsafe or mislabeled goods before trouble hits schools or hospitals.
Looking closer at 4-Morpholinecarbaldehyde through the lens of its CAS number, the core message stands out: accuracy in identification isn’t just about following rules. It’s an investment in safer work environments, smoother scientific progress, and stronger public health. Using CAS 4394-85-8 and promoting that standard in daily routines makes life easier for chemists, buyers, regulators, and eventually everyone who benefits from trusted science. Keeping this system front and center means fewer surprises, less waste, and a lot more certainty for anyone involved with chemicals.
| Names | |
| Preferred IUPAC name | Morpholine-4-carbaldehyde |
| Other names |
4-Formylmorpholine Morpholine-4-carbaldehyde 4-Morpholinealdehyde |
| Pronunciation | /ˈfɔːr mɔːr.fəˌliːn.kɑːrˈbæl.dɪˌhaɪd/ |
| Identifiers | |
| CAS Number | 4394-85-8 |
| Beilstein Reference | 1363680 |
| ChEBI | CHEBI:18975 |
| ChEMBL | CHEMBL163268 |
| ChemSpider | 66302 |
| DrugBank | DB04241 |
| ECHA InfoCard | 19e88c0d-b744-4995-81eb-5e460c458eaf |
| EC Number | EC 211-615-3 |
| Gmelin Reference | 34314 |
| KEGG | C06325 |
| MeSH | D019348 |
| PubChem CID | 12514734 |
| RTECS number | MU7700000 |
| UNII | 2OX68607HF |
| UN number | UN3439 |
| CompTox Dashboard (EPA) | DTXSID7033687 |
| Properties | |
| Chemical formula | C5H9NO2 |
| Molar mass | 115.14 g/mol |
| Appearance | colourless to light yellow liquid |
| Odor | sweet amine-like |
| Density | 1.14 g/cm3 |
| Solubility in water | soluble |
| log P | -0.32 |
| Vapor pressure | 0.3 mmHg (25°C) |
| Acidity (pKa) | 8.40 |
| Basicity (pKb) | 2.9 |
| Magnetic susceptibility (χ) | -52.52·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.5120 |
| Dipole moment | 1.75 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 322.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -220 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -448.6 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319 |
| Precautionary statements | Precautionary statements: P261-P280-P305+P351+P338-P304+P340-P312 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | > 131°C |
| Autoignition temperature | 170 °C |
| Lethal dose or concentration | LD50 oral rat 1210 mg/kg |
| LD50 (median dose) | LD50 (median dose): 500 mg/kg (Rat, oral) |
| NIOSH | KL2975000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 1 g/L |
| Related compounds | |
| Related compounds |
Morpholine Acetaldehyde Formaldehyde |