For decades, the story of 4-Methylthiazole-5-Carboxylic Acid has unfolded quietly in research journals and laboratory notebooks. This compound traces its roots back to the middle of the twentieth century, when chemists started exploring heterocyclic chemistry with more curiosity and purpose. It’s tough to ignore the significance that thiazole rings have had since their discovery in the late 1800s by Hantzsch and his peers; their work paved the way not only for studying naturally occurring substances but also for creating compounds that resemble biomolecules. As pharmaceutical companies and agrochemical developers began hunting for new building blocks in the 1960s and 1970s, acid-functionalized thiazoles like 4-Methylthiazole-5-Carboxylic Acid caught a few clever eyes. Universities and industry labs quietly built up syntheses and methods of isolation, steadily documenting their results. This low-profile development has shaped current methods, applications, and safety data surrounding this little heterocycle.
In daily work with fine chemicals and specialty building blocks, you notice how compounds such as 4-Methylthiazole-5-Carboxylic Acid end up on your desk more often than you might think. You find small glass vials, powdery and yellow, waiting for use in advanced synthesis projects. This compound acts as a versatile intermediate. It's become lodged in the toolkits of medicinal and crop science researchers, especially those navigating the complex pathways of heterocyclic chemistry. The thiazole ring, with its sulfur and nitrogen atoms, brings reactivity while the methyl and carboxylic acid groups let researchers tailor interactions in broader molecular structures. Whether in preliminary screening or refining lead candidates, chemists draw upon its properties to carry out innovative transformations.
Pulling a sample out of storage, you see 4-Methylthiazole-5-Carboxylic Acid as a slightly yellow crystalline powder. The solid feels gritty when rubbed between glass, resisting moisture in a way that helps during weighing and storage. With a molecular weight around 143.15 g/mol, most spectra show strong signals for thiazole ring hydrogens as well as the methyl group. The compound melts in the ballpark of 180–185°C, though trace impurities can move that a bit. Its acid function displays a pKa near 4.6, so most buffers at physiological pH would leave it deprotonated. The molecule dissolves with some coaxing in polar solvents like dimethyl sulfoxide and in hot water, struggling in less polar media. Chemists who encounter stubborn solubility problems often resort to slight heating or mild base addition.
Ordering 4-Methylthiazole-5-Carboxylic Acid from a catalog or chemical supplier, you check CAS number 5326-84-5 to avoid mix-ups with similar thiazole acids. Reputable vendors provide detailed COAs that highlight purity, typically over 98% by HPLC, and include chromatograms or NMR spectra to back up their claims. Impurity profiles matter most for pharmaceutical companies, but even academic labs need tight controls to get clean experimental results. Labels warn of irritancy and suggest using gloves and goggles. You rarely see it shipped without documentation about disposal, handling, or storage practices, reflecting years of regulatory evolution and the growing need to keep lab work safe and traceable.
If you’ve ever tried to make 4-Methylthiazole-5-Carboxylic Acid in the lab, you learn quickly that classic Hantzsch synthesis routes rarely give you high yields in a single step. Most protocols start from thioamides or methyl-substituted thiazoles, often reacting these with oxidizing agents alongside suitable carboxylation steps, using CO2 or related reagents. Catalysts make a difference; copper and palladium complexes have edged into procedures reported in recent years. Older literature suggests route tweaks using acyl chlorides or malonates, though these need careful attention to temperature and pH control. The scale-up from milligram to gram quantities inevitably forces changes—extra purification, maybe a switch from liquid-liquid extraction to preparative chromatography. Yields often hang in the range of 60–80%, with several washes, filtrations, and crystallizations built in along the way.
The chemistry of 4-Methylthiazole-5-Carboxylic Acid keeps pushing boundaries, especially in medicinal chemistry, where every functional group earns its keep. The carboxylic acid group opens the door to forming esters, amides, and acyl chlorides, which are then wired into more elaborate molecules. Hydrogenation of the thiazole ring doesn’t get much attention because aromaticity gives a sharper edge to its biological activity, but various groups have attempted halogenations, nitrations, and even Suzuki couplings, leveraging the methyl group’s reactivity at C-4. You see the impact of these modifications in the library compounds submitted for high-throughput screening—slight tweaks at the methyl or carboxyl position generate analogs with altered binding or metabolic profiles.
Industry rolls out several names for the same molecule, leading to its fair share of confusion. You look through catalogs or patents and see 4-Methyl-5-thiazolecarboxylic acid and 4-Methylthiazole-5-carboxylic acid side by side. Labs working on inventory management track this acid under abbreviations such as MTCA. Lists in chemical inventories also log numbers like its EC Number 226-247-5, while some databases cross-link it to CompTox and PubChem IDs, smoothing out information flow between groups. Keeping track of synonyms helps when following up on old research that used dated nomenclature; clear naming speeds up the translation from literature to practice in research.
Handling 4-Methylthiazole-5-Carboxylic Acid does not feel dramatically risky, but it demands basic caution. Most labs store it in tightly sealed containers, away from heat and ignition sources. Dust can irritate skin, eyes, and respiratory tracts, calling for gloves, safety glasses, and, sometimes, working under a fume hood if weighing out many samples. Chronic exposure data remain limited, but the general culture now leans toward minimizing unnecessary risk, especially with repeated contact. Disposal flows through standard channels—dilute solutions into labeled waste collection, solids collected for incineration. Every reputable supplier packs Material Safety Data Sheets that cover acute hazards and give first-aid advice, a step above the loose standards you’d see a generation ago.
Applied researchers and product developers recognize 4-Methylthiazole-5-Carboxylic Acid as much more than a bench curiosity. Medicinal chemists use it to build antifungal or antibacterial scaffolds, betting on sulfur-nitrogen functionality to hit novel binding pockets or disrupt enzymes. Agrochemical groups plug its thiazole core into seed coating experiments or pesticide prototypes, chasing selectivity with less environmental fallout. Polymer chemists and material scientists spot the potential in advanced conjugations, giving new life to electronic or optoelectronic research. Even flavor chemists take a sideways look at this class of heterocycles—though methylthiazoles often end up in roast flavors, the carboxylic acid falls more naturally into analytical standards than consumer products. Each industry claims a slice, and demand tracks the flow of seeds, screens, and patents as the seasons turn.
Research into 4-Methylthiazole-5-Carboxylic Acid ramps up in cycles—booming when some pharma startup plows through a fragment library or boosting again when public funding pivots toward new antimicrobial agents. Academic groups tool up with fresh catalysts to streamline carboxylation or tweak reaction times. Journals publish dense NMR and mass spectrometry data; online chemical marketplaces echo these trends with new product launches and purity grades. Collaboration between public and private labs pushes standards higher and brings automation into synthesis. Open-access chemical libraries make new analogs easier to share, and the flood of preprints ensures you rarely lose touch with what's happening. If you have ever presented at a conference, you know a single modification on the thiazole ring can spark ten conversations and at least a few friendships.
Direct toxicity data on 4-Methylthiazole-5-Carboxylic Acid remains sparse, though thiazole derivatives have drawn enough scrutiny over possible cytotoxic and teratogenic effects to merit careful handling. Lab-based studies in the past 20 years focus on comparative animal models or cell lines, looking for red flags with repeat dosing or metabolic buildup. Few studies document acute oral or dermal effects in detail, but most regulatory filings suggest mild to moderate irritancy. Chronic exposure data are lacking, pushing safety-conscious labs toward minimizing waste and maximizing PPE usage. Data on biodegradation point to slow breakdown under aerobic conditions, flagging the compound for special handling in environmental contexts.
Looking ahead, 4-Methylthiazole-5-Carboxylic Acid waits at a crossroads of expanding applications and tightening safety oversight. Automation stands ready to boost production and consistency. AI-driven reaction planning feeds directly into route optimization, letting labs reduce steps and cut down waste streams. Life sciences innovators draw more and more attention to heterocyclic acids like this one, so the compound stands a good chance of turning up in next-generation antibiotics or niche agrochemicals. Safety data will keep moving forward, especially as regulatory boards raise the bar for environmental disclosures. If industry and academia communicate smoothly, streamlined methods and greener chemistry could turn the next decade into a period of accelerated discovery and improved stewardship of molecules like 4-Methylthiazole-5-Carboxylic Acid.
Let’s get straight to the facts: 4-Methylthiazole-5-carboxylic acid carries the chemical formula C5H5NO2S. Its molecular weight checks in around 143.16 g/mol. Those numbers might seem like details that chemists toss around just for fun, but in my own lab experience, accuracy with these basics separates a sloppy experiment from a successful one. Mess up a formula, and you might waste precious weeks or worse—draw the wrong conclusions.
I remember wrangling with a tricky synthesis back in grad school where a thiazole derivative kept showing up as a contaminant. The tiniest mistake in reporting its structure caused a chain reaction of confusion down the line. It’s easy to dismiss small heterocycles like this one—after all, thiazoles pop up in everything from vitamins to agricultural fungicides. But 4-Methylthiazole-5-carboxylic acid has carved a surprising niche. Its structure combines a sulfur and nitrogen in the ring, a methyl group, and a carboxylic acid. That mix creates a molecule that can slip into many different biochemical gears, sometimes acting as a flavor enhancer, sometimes serving as a stepping stone for drug candidates.
A lot of chemists, myself included, learn early that formulas aren’t just abstract representations—they’re the recipe. Get a single atom wrong, and you are working with a new substance entirely. I’ve seen mislabeling cause safety concerns and wasted budgets. Precise reporting of C5H5NO2S helps researchers, analysts, and manufacturers avoid these pitfalls. It’s not about memorizing numbers to impress people. It’s about making sure the folks mixing chemicals in industrial vats stay safe and get predictable results.
Molecular weight tells us more than how many grams to weigh out. Labs rely on it to calculate dosages, anticipate how a compound will behave in water, and predict how fast it might zip through a chromatography column. Once, while prepping a new catalyst, the only molecular weight reported for an intermediate was off by two grams. That error caused weeks of failed recrystallizations and a whole lot of headscratching. If you run a business or a research group, every mistake like this erodes trust and eats up resources.
I’ve found that triple-checking numbers before submitting a report saves hours down the road. Open collaboration tools and standardized databases like PubChem have helped cut down confusion about chemical identities. If more researchers build these checks into their routines and keep formulas easy to cross-verify, labs will see fewer slip-ups. Investing a little bit of time up front in training students or new hires also pays huge dividends; it turns out that most misunderstandings about a compound’s identity can be avoided just by revisiting the basics.
Chemicals like 4-Methylthiazole-5-carboxylic acid might not get headlines, but their reliability helps everything from medicine to food flavorings progress safely. Beneath every successful formulation sits someone who sweated the details: the right formula, the correct molecular weight, and an eye trained to spot where things could go wrong. That’s how we move from good intentions to dependable results—and it all starts with these backbone facts.
4-Methylthiazole-5-carboxylic acid doesn’t turn many heads outside chemistry circles. It doesn’t show up in viral videos or sit beside household cleaners under the kitchen sink. Still, for people who spend time in a chemical lab or in research, it’s a familiar backbone, a steady ingredient that unlocks a surprising range of functions, mostly where building blocks matter more than big headlines.
This molecule stands out for its utility in making medicines. Drug chemists often look for pieces that stick well to other structures and deliver helpful properties. 4-Methylthiazole-5-carboxylic acid delivers just that. Thiazole rings show up in plenty of medicine cabinets, tucked inside antibiotics, anti-inflammatory products, and antidiabetic treatments. This building block blends into these bigger molecules, providing a unique twist that drug designers appreciate. The ring adds stability and can help tune how drugs interact with the body.
One big reason to care about precursors like this one: they help drug makers adjust effectiveness and reduce harmful side effects. For instance, thiazole groups commonly show up in penicillins, cephalosporins, and newer anti-infectives. Chemists can swap this piece in and out, changing how a medicine works. Nobody wants a medicine that clears up an infection but knocks out the kidneys in the process. With the right building pieces, scientists nudge drugs in the right direction.
While folks focus a lot on health, agriculture leans on this acid, too. The molecule finds its way into new agrochemicals, especially pesticides and herbicides. It does well helping manage plant diseases or bugs that threaten crops. Companies searching for next-generation solutions keep a close eye on how thiazole-carboxylic groups help their formulas work. New research out of Germany and Canada often discusses these structures, as farmers keep asking for smarter tools that balance safety and yield.
In my brief chemistry work after college, we always aimed for powerful but safe formulas. 4-Methylthiazole-5-carboxylic acid catches the eye as it helps keep pesticide potency high but toxicity manageable—a real concern for food producers and the planet.
Chemical research never stands still, and this acid’s role shouldn’t get overlooked. Thiazoles—especially as carboxylic acid derivatives—act as useful stepping stones to more complex chemicals. In material science, that might mean new polymers for electronics or coatings. Research articles out of Asian universities describe pilot projects where this molecular piece plays a role in optoelectronics and photoactive materials.
Patents over the past five years list this acid as a critical component in chemical libraries for screening new compounds. Every time a researcher needs a reliable, reactive building block for synthesis routes, compounds like this show up in their notebooks.
All of this comes with responsibility. Thiazole derivatives can raise environmental flags if not handled with care. As the chemical industry gets stricter about regulations, having clear pathways for safe manufacturing and waste treatment grows even more important. Research groups across Europe publish studies on biodegradability and safer production methods, tying into the rising demand for green chemistry.
My own experience handling chemicals reminds me: easy-to-make doesn’t always mean easy-to-clean. Solutions don’t just lie in high-tech gear, but in smart design and industry transparency. The more companies invest in both new applications and greener pathways, the better long-term value everyone receives—from scientists and farmers to everyday consumers.
Working with chemicals like 4-Methylthiazole-5-Carboxylic Acid offers progress and new products, but it also brings risk. I’ve spent enough time around hardworking chemists and plant workers to see that details matter more than labels suggest. This isn’t some mystery powder you can just stash away. Whenever I’ve visited labs—big-budget or the high-school kind—you could always spot the difference: the places running a tight ship had fewer spills, fewer headaches, and sometimes even a better story to tell at the end of the day.
Glass bottles or chemical-resistant plastics win here. Steer clear of old food jars or metal cans; those corrode or contaminate fast. Even the best chemical loses its edge if left in a flimsy bag that fails during a humid spell. I once watched a whole week's work—gone—because of a plastic lid that cracked overnight. The right material guards against leaks, keeps the air out, and, most importantly, cuts down on unpredictable reactions. Not every workspace can afford the fanciest gear, but a decent, tight-sealing cap and a clean, dry shelf go a long way.
Folks often rush through labeling, thinking it’s unnecessary. Having seen my share of mystery bottles, I can tell you: it’s not worth the risk. A clear label, showing name, date, and any hazards, prevents confusion. It protects people who didn’t prepare the original sample and shields businesses from headaches during inspections. In my earliest days, I once had to sort through a dozen barely marked vials. It slowed us to a crawl and made disposal trickier—and costlier—than it should’ve been.
Most chemical guides urge cool, dry storage, but the details matter. If the space feels muggy or hot—think attic or boiler room—you’ll get clumps, spoilage, or weird odors. Climate control pays off. I recommend keeping this compound below 25°C and away from direct sunlight, since UV can change the game. I’ve seen labs install affordable temperature monitors that beep if it gets too warm. This trick can save batches worth hundreds or thousands.
Putting acids, bases, or oxidizers too close together spells trouble. One place I worked lumped all white powders on a shelf, and a dropped container caused a foul-smelling mess from a surprise reaction. Lock away incompatible materials. If space runs short, simple barriers work: even a sturdy cardboard divider can help in a pinch, though metal or fireproof cabinets do better for larger stocks.
Goggles, gloves, and sometimes an apron don’t just keep up appearances. They keep skin safe and eyes healthy. Even a light dusting from the wrong chemical gets under your nails and lingers; I learned this the hard way clearing a jammed dispenser. Regular washing and not eating near your work area go farther than folks realize.
Proper handling isn’t only about safety manuals. It also extends shelf-life and preserves quality. I’ve watched experienced workers take tiny scoops with clean, dry tools—it keeps the rest of the bottle safe for next time. Resealing bottles right after every use matters more than most folks think.
Once finished, don’t dump leftovers down the sink. Chemical waste handlers know how to neutralize and store this material for collection. Teaching new employees these steps beats retraining every few months.
Thoughtful storage and careful handling don’t just keep everyone safe—they save money and keep projects on track. In my experience, the labs and workshops that thrive take these small steps every day.
Purity in chemicals matters more than most people outside the lab might guess. With something like 4-Methylthiazole-5-Carboxylic Acid, the cleaner the sample, the fewer chances for headaches down the road. Whether you’re outside the chemistry world, you can probably imagine that shaky quality can throw a wrench into things. Think of building a bookshelf with warped wood; measurements stop making sense, and you start to wonder what went wrong. Scientists and manufacturers who use compounds for research, drug development, or specialty chemicals ask for clear information on purity. Nobody wants contaminants or mystery ingredients sneaking into their work.
High purity isn’t just a marketing term. In pharmaceuticals, for example, even a tiny impurity could change how a drug acts. I remember a small lab I visited years ago. They spent months on a project, only to discover a surprise impurity in a reagent. They had to toss almost everything and start from scratch. A costly lesson, but not an uncommon one. For 4-Methylthiazole-5-Carboxylic Acid, research grade often runs above 98% purity, particularly prized for R&D, fine organic synthesis, or drug candidate development. Technical or industrial grade comes with more leeway on impurities, sometimes in the 90–95% range, because the stakes in manufacturing dye batches or flavor components differ. Quality control relaxes when applications don’t call for absolute precision.
If buyers want to choose, it means keeping a close eye on the source. The big chemical suppliers sometimes offer a handful of purity options and list them in their catalogs. Anyone browsing these will notice purity levels labeled front and center: “analytical grade,” “technical grade,” sometimes even “pharmaceutical grade.” I’ve seen some labs haggle with suppliers to provide up-to-date Certificates of Analysis for each batch, making sure that the product hasn’t changed between orders. This can be tedious, but no one likes the idea of running a batch of tests and then learning they got the wrong grade—one with too much gunk sneaking in.
There’s always a battle between the need for high purity and the reality of budgets. Ultra-pure samples get pricey fast, which can stop small groups in their tracks. Sometimes labs settle for less—especially in early-stage work or pilot batches—then splurge for higher purity once a project picks up speed. Chemical manufacturers know this and try to offer a suite of options. It’s a trade-off every buyer faces: pay up front for reliability, or roll the dice and hope lower purity works out.
A little more transparency would make life easier for everyone. Consistent labeling, reliable documentation, and clear communication from producers could cut down on last-minute hiccups. There’s room for smarter digital systems, where buyers could track the origins and certifications at a glance. Making purities and grades easier to compare also helps smaller buyers—academics or startups—who don’t have the time or staff to chase down chemical paperwork all day. As technology creeps forward, automation and testing advances could lower the cost for higher purities, opening new doors for research and manufacturing.
Anyone who’s spent time in a lab or production plant knows that chemicals don’t play by friendly rules. 4-Methylthiazole-5-Carboxylic Acid, for example, might not sound like a household hazard, but underestimating it catches people off guard. I’ve watched smart scientists and eager lab techs learn this the hard way. To avoid that, treating every new compound with an extra dose of caution makes sense.
Never trust a bare hand with chemical powders. Disposable nitrile gloves protect skin, but changing them with each new task helps. Splashing even a small amount in your eye is easy if safety goggles aren’t in place. There’s no badge of honor for pushing through without protection. No one remembers the report you finished quickly, just the accident you could have prevented by grabbing that gear. I’ve kept my own goggles in a lab coat pocket for years now—often a habit that keeps the crisis away.
Fume hoods and extraction fans save more than your sense of smell. 4-Methylthiazole-5-Carboxylic Acid may cause irritation or worse when inhaled. Fans running across the bench never clear up harmful vapors. A real fume hood does the heavy lifting, so keeping the sash down just enough for your hands blocks splashes and traps fumes before they become a problem. It’s tempting to cut corners here—everyone does now and then—but every stack of safety data sheets backs up the value of real ventilation.
Sharpies and labels exist for a reason. Mixing up or forgetting a container makes accidents more likely than anything else in the workroom. With enough experience, grabbing samples out of habit takes over. A plain flask with a hand-written label saves you from losing product batches, or worse, triggering a bad reaction later on. More than one colleague has swapped acids and ended up cleaning up a mess that could have sent them to the ER.
Spills stop work and set everyone on edge. Planning for accidents means keeping spill kits stocked: absorptive pads, neutralizers, and disposal bags all have their place. I’ve learned that catching a small spill with the right powder or granule beats scrambling for paper towels and water. Once you’ve seen a spill spread under a stack of research notes, or migrate under another piece of equipment, over-planning doesn’t feel like a joke.
Material Safety Data Sheets (MSDS) often sit unread on a shelf—or buried in some computer folder. Taking ten minutes to read through the hazards, storage limits, and first aid steps means fewer surprises. 4-Methylthiazole-5-Carboxylic Acid isn’t rare enough to ignore, and important details get lost if no one knows the right page to check in an emergency. I like to quiz new team members on these bullet points. It’s worth looking a bit overbearing if it saves somebody from a trip to the medical office.
Tossing chemicals in the wrong waste bin is how a regular day turns bad quickly. 4-Methylthiazole-5-Carboxylic Acid deserves a proper place: designated acid waste bottles with clear labels. Chemical waste doesn’t just disappear. Even when busy, it pays to double-check. Local environmental teams audit disposal habits often, so keeping track from the start builds good habits that stick with you everywhere.
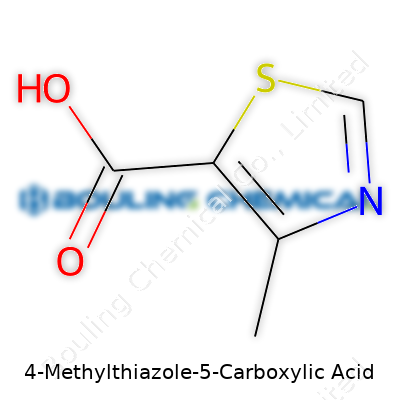
| Names | |
| Preferred IUPAC name | 4-methyl-1,3-thiazole-5-carboxylic acid |
| Other names |
4-Methyl-1,3-thiazole-5-carboxylic acid 4-Methylthiazole-5-carboxylate 4-Methyl-5-thiazolecarboxylic acid 4-Methyl-5-thiazolecarboxylate |
| Pronunciation | /ˈfɔːr ˌmɛθ.əl θaɪˈæz.oʊl faɪv kɑːrˈbɒk.sɪl.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 53298-51-6 |
| 3D model (JSmol) | `3D model (JSmol)` string for **4-Methylthiazole-5-Carboxylic Acid**: ``` CC1=NC=C(S1)C(=O)O ``` This is the **SMILES** string, commonly used in JSmol and other molecular visualization tools. |
| Beilstein Reference | 1582083 |
| ChEBI | CHEBI:91263 |
| ChEMBL | CHEMBL463330 |
| ChemSpider | 23736958 |
| DrugBank | DB08347 |
| ECHA InfoCard | 03b8b7a7-4695-46fc-bbe4-9c8d6eacef7d |
| EC Number | 872-003-00-2 |
| Gmelin Reference | 10430 |
| KEGG | C06188 |
| MeSH | D058424 |
| PubChem CID | 697301 |
| RTECS number | VW2684600 |
| UNII | Q1Q0TGC65E |
| UN number | Not classified as dangerous goods |
| CompTox Dashboard (EPA) | DJ55X9T533 |
| Properties | |
| Chemical formula | C5H5NO2S |
| Molar mass | 143.16 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.41 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 0.11 |
| Acidity (pKa) | 3.2 |
| Basicity (pKb) | 6.29 |
| Magnetic susceptibility (χ) | -23.1 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.586 |
| Dipole moment | 2.71 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 181.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -184.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -411.9 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, causes skin irritation |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | Flash point: >110°C |
| NIOSH | PB9187000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m3 |
| IDLH (Immediate danger) | NIOSH does not list an IDLH value for 4-Methylthiazole-5-Carboxylic Acid. |
| Related compounds | |
| Related compounds |
Thiazole 4-Methylthiazole Thiazole-5-carboxylic acid 2-Methylthiazole Thiazole-4-carboxylic acid 5-Methylthiazole 4-Methylthiazole-2-carboxylic acid |