The roots of 4-methylpiperidine stretch back to the twentieth century, at a time when research into nitrogen-containing heterocycles fueled the growth of pharmaceutical chemistry. Chemists found that small tweaks, like adding a methyl group on the piperidine ring, change physical properties and even make certain chemical reactions more practical. The knowledge didn’t come from just academic curiosity. During the early wave of drug discovery, variations on simple piperidine shapes created whole classes of compounds, each with its set of uses and surprises. The ramp-up in industrial synthesis during the late 1960s brought larger volumes of 4-methylpiperidine, making it a tool not only for researchers but also for manufacturers hungry for specialty intermediates.
4-Methylpiperidine pops up in drum labeling as a clear, colorless to pale yellow liquid with a peppery, distinctly amine-like odor. In everyday lab scenarios, suppliers offer it by the liter or kilogram, sometimes stabilized with small amounts of copper or other inhibitors to prevent discoloration. If someone is working with flavorings, polymers, or setting up a synthesis run for complex molecules, they’ll find 4-methylpiperidine can change the outcome of a process in unexpected ways. Its accessibility in both bulk and research quantities makes it a mainstay, but the real draw lies in the way it helps unlock more complex chemistry downstream.
Anyone who’s uncapped a bottle of 4-methylpiperidine knows its strong, sharp smell can clear a room. It boils at about 106-107°C, has a melting point way below zero, and shows classic water-miscibility at moderate temperatures. Unlike many of its relatives, that methyl group at the four position changes solvent behavior, slightly increases its basicity, and makes it handy in liquid formulation work. Chemical stability holds up well under common storage conditions, although exposure to strong oxidizers or sunlight over time can turn the liquid darker and less pure. In practice, pH in water lands well above neutral, a trait prized by chemists needing a strong, non-aromatic organic base.
Suppliers print out the critical details — molecular formula C6H13N, CAS number 626-58-4, boiling point, density, flash point. Purities above 98% are standard for most research needs, sometimes labeled “anhydrous” or “distilled” for higher spec work. Containers bear the hazard diamond warning for flammability and health risk, and every shipment comes with a safety data sheet. Labels emphasize the need to avoid breathing vapors and require gloves and goggles at the bench. Large drums for scale-up batches need proper venting since vapors can build up and escape fast in warm rooms.
Large-scale factories and university labs stick to the same few synthetic routes. One practical pathway starts from piperidine, using methylation — often with methyl iodide or dimethyl sulfate — followed by purification to remove byproducts. Another route takes cyclization of 4-methylamine-1-butanol, looping the chain into the six-membered ring. Each route brings its own quirks, waste streams, and purification headaches. Production teams often fine-tune batch parameters to cut back on residual salts or hard-to-remove side products that can plague downstream chemistry.
4-Methylpiperidine plays a role in more than just one type of reaction. Researchers reach for it as a non-nucleophilic base, especially in situations where other bases cause too much trouble. In Mannich reactions, it can act both as a reactant and as a catalyst to drive addition products. Medicinal chemists often attach functional groups at the nitrogen or tweak the methyl position to dial up potency or metabolic stability in test compounds. Oxidation, acylation, and reductive amination procedures often include 4-methylpiperidine as either a nucleophile or a base, reflecting its adaptability and broad reactivity.
A search for 4-methylpiperidine turns up more identities than you’d expect: N-methylpiperidine-4, 4-methyl-hexahydropyridine, and even simple “MPIP” on drum labels. International companies sometimes go with “Piperidine, 4-methyl-” just to keep customs paperwork smooth. Each synonym follows a thread through catalogs, research databases, and safety inventories, ensuring that chemists in different labs track down the same compound despite all the name changes.
Work with 4-methylpiperidine runs best under a fume hood and with gloves, thanks to its volatility and skin-wicking tendencies. Accidental spills cause more than a sharp odor — they hit eyes and skin with irritation, and vapor inhalation stays unpleasant for hours. Safe storage means tightly sealed bottles, away from acids and oxidants, at room temperature or a slight chill. Every scale-up batch urges extra caution because its low flash point spells fire risk if left around open flames or electrical sparks. Waste streams need neutralization before disposal. Most labs keep calcium chloride or similar drying agents nearby since the compound likes to pull in water if left in the open.
The reach of 4-methylpiperidine covers far more than textbooks suggest. Pharma companies value it for building piperidine-based drug candidates, especially for targeting neurological and anti-inflammatory pathways. Polymer manufacturers slip it into specialty plastics as a catalyst and chain modifier for higher performance. Some flavor chemists note its low-level presence in certain tobacco products, helping tweak aroma or mouthfeel. Analytical chemists use traces as standards for fine-tuned calibrations. Chemical researchers keep bottles on hand for organic synthesis, studying the impact of minor ring changes on everything from liquid crystals to pesticide analogues.
Pushes to optimize reactions have launched hundreds of studies into the quirks of methylated piperidine. Faster, greener methylation methods – swapping out toxic reagents, minimizing waste, boosting yields – pop up in academic journals every year. Drug design teams search for more selective ways to build on the 4-methyl group, exploring its role in receptor binding and metabolic breakdown. Computational chemistry helps map how 4-methylpiperidine stacks up in basicity, electron distribution, and ring strain, letting researchers predict reactivity before even heading to the bench. Scale-up specialists work to cut down residual contaminants and energy input for making metric tons a year, chasing both cost savings and lower emissions.
Years of toxicity work show that 4-methylpiperidine, like many small amines, acts as both an irritant and a possible systemic toxin if inhaled or ingested in larger doses. Skin contact brings stinging or redness, and animal studies suggest neurotoxic effects at higher chronic levels. Workplace exposure limits remain tight, echoing regulatory bodies' push to safeguard workers handling volatile organics. Environmental fate studies flag slow biodegradation and some risk for aquatic systems if spills go unchecked. Labs follow strict protocols for spills and waste, and routine health monitoring of workers catches signs of cumulative low-level exposure.
The future for 4-methylpiperidine grows brighter as its toolkit expands. Drug developers see it as a springboard for next-generation painkillers, antidepressants, and immune modulators, building on the structure to drive selectivity and cutting side effects. Green chemistry keeps pushing for better, safer routes — swapping out hazardous methylating agents and designing closed loop systems that reuse solvents and cut waste. Polymers, specialty coatings, and advanced materials industries look for new roles, especially as the world demands higher performing, more sustainable chemicals. As regulations evolve, both safety and production standards will adapt, carving out space for both innovation and responsible stewardship of this key nitrogen compound.
4-Methylpiperidine rarely grabs headlines or inspires dramatic exposés. Yet, in the world of chemistry, plenty of unsung compounds quietly hold up bigger stories. You can find this small molecule behind the scenes, shaping everything from pharmaceutical research labs to coatings on factory tools. Not the sort of thing you’d expect to talk about over dinner, but absolutely the kind of substance that keeps certain wheels turning without fuss.
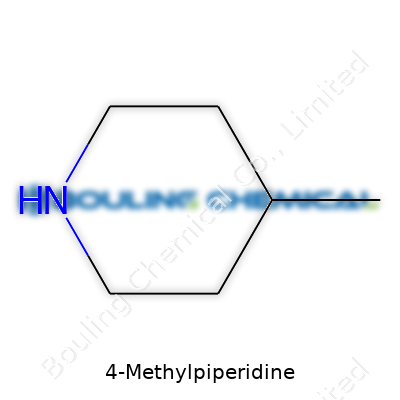
It starts with the structure—a six-membered ring with nitrogen and a methyl group sticking out. You won’t see it sold on pharmacy shelves or at the corner hardware store. Chemists, on the other hand, reach for it to help steer complex reactions. In the synthetic lab, this compound helps scientists adjust pH levels or coax out specific reactions that wouldn’t go anywhere without a base.
Drug development today relies on complicated pathways and fine-tuned ingredients. Researchers saw long ago that certain nitrogen chemicals could smooth out reaction pathways, making pills and injections safer and more efficient to produce. 4-Methylpiperidine often steps in as an intermediate or a starting point. For example, it lends itself to the manufacture of drugs for treating everything from mood disorders to infections. This isn’t just hypothetical—patent filings document it cropping up in routes toward antidepressants, analgesics, and even anti-cancer treatments. The people working long hours in R&D know what they get with this chemical: reliability, specificity, and a helpful hand in the transformation from simple to complex.
Not every task lands in the pharmacy aisle. Factories and refineries also hunt for chemicals that won’t break down under pressure or temperature. 4-Methylpiperidine’s sturdy, amine backbone can kick off chain reactions in resins and polymer production. Think about paints clinging longer to metal parts or protective coatings shielding electronics in humid climates. If you own a gadget that survives the elements a little longer, odds are that somewhere along that device’s assembly line, a piperidine derivative stepped in to help out.
Every chemical that enters industry brings along environmental and safety questions. 4-Methylpiperidine doesn’t escape scrutiny here. Exposure can cause irritation, and accidental spills come with real hazards for workers and communities. Some countries list it as a hazardous substance, putting extra requirements on storage and disposal. I’ve seen facilities where improper chemical handling turns routine days into emergencies, so it’s worth pushing for tighter protocols—training programs, investment in containment technology, and the switch to greener alternatives when suitable.
There’s always a race to outpace risk through better design and regulation. Researchers now probe bio-based amines and improved recycling systems to handle older, less friendly chemicals. Meanwhile, engineers in the field juggle cost, safety, and availability every day, sometimes swapping out traditional compounds like 4-Methylpiperidine for newer materials when regulations or market forces nudge them. The world can’t stop using chemistry, but it can demand more from those who design, ship, and clean up after these processes.
Most people hardly glance at the name 4-Methylpiperidine and think twice. I used to lump names like that together—just labels for chemicals you probably don’t keep around the house. But every chemical has a story, woven right into those few squiggly lines scientists use to draw them. In the case of 4-Methylpiperidine, that story starts with its chemical formula: C6H13N. That “C” means carbon, “H” means hydrogen, and “N” is nitrogen. Mix six carbons, thirteen hydrogens, and one nitrogen, and you get this one particular ring-shaped compound.
Chemistry’s some people’s daily grind, but most folks interact with these invisible molecules only when they matter—a new lab discovery, a worrying news story, or some label on cleaning supplies that isn’t in plain English. Knowing what sits behind names strips away that mystery. I remember a time I stumbled through a university lab, staring blankly at bottles, sure that every syllable spelled danger. Later I realized most chemicals, including 4-Methylpiperidine, spend their time helping to build other stuff rather than causing harm.
Now for the number-crunching part. Molecular weight doesn’t earn headlines, but it’s vital. 4-Methylpiperidine clocks in at around 99.18 g/mol. That number comes from adding up the individual atomic masses: each carbon weighs about 12.01, each hydrogen clocks in at about 1.008, and nitrogen weighs roughly 14.01. Stack them up the way the formula tells you, and you’ve got your weight. It’s the kind of math that looks simple, though it guides all sorts of real-world processes—from dosing medications to manufacturing solvents.
If you’re in a research lab, students probably ask all the time, “Why care about the exact weight?” Measure out too much or too little, and you throw off the whole reaction. Factories churning out paint, pharmaceuticals, or plastics trust those numbers so they don’t waste materials or endanger people. At its core, this is about precision—a lesson most of us could use, regardless of our usual distractions.
For years, chemists have leaned on 4-Methylpiperidine for important reactions like making pesticides, medicines, or flavor compounds. Reading safety data sheets and ingredient lists keeps you awake to the impact these single molecules pack—positive and negative. Some compounds get a bad rap, but a lot of that comes from not having context. I remember the sense of relief in students’ faces when we’d decipher a tricky name and realize it was a building block, not a poison.
Still, we don’t live inside a chemistry textbook. Mishandling even everyday chemicals can lead to leaks, contamination, or worse. Regular folks, not just scientists, need access to clear, direct facts rather than codes and jargon. We could do more to link chemical literacy to everyday safety—something as basic as requiring plain English translations on chemical packaging could shrink the number of accidents. That’s as true for 4-Methylpiperidine as it is for bleach or ethanol.
Bringing more sunlight to chemical information helps the whole system. Industry folks, students, and even parents who just want to keep their households safe all win when products label building blocks clearly. For 4-Methylpiperidine, just knowing it’s C6H13N with a molecular weight of 99.18 g/mol already puts control back in ordinary hands. Nobody needs to stay in the dark—or risk a preventable mistake—when the tools for understanding are built into the formula itself.
Ask anyone who’s worked in a small lab or a big chemical plant and they’ll have a few stories about 4-Methylpiperidine. While it’s not exactly a household name, in the labs, its presence is hard to ignore—a liquid with a sharp, almost fishy tang that lingers in the air far too long. One slip in handling or storage, and your workspace stinks for days. It's not just about comfort; poor storage brings real risks to health and safety.
A lot of folks new to chemical work might figure it’s fine to just stash a bottle of 4-Methylpiperidine on a shelf. Experience tells a different story. This chemical catches fire easily. Its vapors—heavy, persistent—don’t wait around politely near their container. Uncapped, even for a short time, and your nose knows about it before your eyes do. Storing it in a flammable liquids safety cabinet, well away from ignition sources, cuts down on risk. The standard walk-in lab fridge isn’t the right spot, either. The chemical doesn't like moisture—a leaky seal means water in the air, which can mess with the quality inside the bottle and make things more dangerous.
Labeling feels dull, but it saves lives. In my first job at a startup, mismarked bottles caused so many headaches. 4-Methylpiperidine’s volatility means everyone needs to know exactly what’s inside each container. A clearly marked, tightly sealed vessel (often amber or metal) lets coworkers avoid dangerous mix-ups. Keeping a register of all containers in the lab, checked at least every few weeks, helps spot leaks before they become emergencies.
I once watched a coworker pop open a bottle in a cramped storeroom and nearly pass out. One whiff of concentrated vapor can leave you reeling. Good ventilation keeps the air moving and toxins low. In most labs and chemical stores, using a chemical fume hood or storing materials in a vented cabinet balances productivity and safety. Skipping this step risks not only workplace safety but also contributes to that long-term, lingering smell that never fully leaves your clothes or hands.
Gloves and goggles don’t just look professional—they block the worst the chemical tries to do to your skin and eyes. Spills dry out skin, sting eyes, and in strong concentrations, lead to chemical burns. Soap and water on standby aren’t a luxury—they’re part of daily life where 4-Methylpiperidine gets handled. It makes sense to check for leaks or damaged caps before moving any container. Shortcuts can turn a small spill into a full evacuation.
People make mistakes under stress. Having clear guidelines and regular walk-throughs helps the whole team stay sharp. Each person’s work habits set the tone for those around them. Short safety talks, quick walkthroughs, and simple double checks teach everyone what safe storage and handling really mean. A strong safety culture turns the storage of chemicals from a worry into a routine task, keeping everyone safer and turning what looks like a hassle into the mark of a team that pays attention to what matters.
Most people won’t have heard of 4-Methylpiperidine unless they’ve spent time in a chemistry lab or worked with industrial chemicals. This stuff plays a small but important role behind the scenes in pharmaceuticals, agrichemicals, and even some rubber production. Getting too comfortable around chemicals with confusing names can blur the line between routine work and real danger. Personal history has taught me it’s usually the unfamiliar compounds, not the infamous ones, that trip people up.
4-Methylpiperidine brings more risk than the average cleaning solution or household solvent. Most forms come as a clear, strong-smelling liquid. The smell gets to you fast—think of a blend between ammonia and stale fish. If that odor seeps into the air, it means the chemical is out and likely causing trouble already.
Breathing in the vapor—even briefly—can irritate the lungs, make your eyes water, and bring on headaches or dizziness. My hands have tingled before from accidental skin contact, and I’ve seen coworkers rush to eyewash stations after just seconds of exposure. The liquid burns skin, can damage eyes, and definitely won’t do your stomach any good if swallowed. Even mild contact leaves you feeling sore and wishing you’d been more careful. Reports show longer-term exposure can hit the central nervous system, risking confusion or coordination problems, which isn’t what you want in any workplace.
There’s also the fire risk. 4-Methylpiperidine catches quickly at lower temperatures than you’d expect. Spilled on a bench under warm lights, it can light up from something as simple as a spark from a frayed cord.
My time in shared labs and workshops taught me a simple lesson: respect the chemical, not just the warning label. Proper ventilation always matters with volatile compounds. Open windows and a working fume hood aren’t extra steps—they're the basics. Even on rushed days, putting on gloves, goggles, and a lab coat before handling any bottle saves skin and eyes from serious pain. I still remember the sting from a glove with a tiny hole after a careless reach.
Label containers clearly and lock them in flammable storage cabinets when work finishes. Spills can’t wait for “later”; have absorbent pads and spill kits close by, and clean up messes right away. Washing hands becomes second nature if you work with this stuff—it lingers in creases and under nails. Fire extinguishers designed for chemical fires, not just basic powder types, should sit near every spot where flammable chemicals get used. Local safety training paid off for me and my crew during a close call years ago—knowing exactly where to run and what tool to grab means the difference between a scare and a hospital visit.
Transport and dispose of leftovers through licensed companies rather than the regular trash. It takes more time, but skipping steps ends up costing more if someone gets hurt or the company faces clean-up costs. Safety datasheets aren't just there for the paperwork shuffle—they hold specifics about symptoms, treatments, and what to do if things go wrong. Checking those before a new job keeps mistakes to a minimum.
Companies serious about chemical safety teach staff with real-life drills, not just slideshows. Sharing stories about minor mistakes (and what they nearly cost) wakes people up better than rules stuck on the wall. Regular equipment and storage checks lower the odds of surprise leaks. Pushing for more awareness about how chemicals like 4-Methylpiperidine can cause fire, injury, or long-term health problems will save more than just property in the long run.
Ignorance isn’t an excuse. Training, habits, and a bit of respect will keep people whole and healthy even in environments that seem predictable. Every person handling 4-Methylpiperidine holds the next shift’s safety in their hands, whether they work in a towering plant or a backroom lab.
Ask any lab technician or procurement officer about 4-Methylpiperidine, and you might notice the same look of resignation—they either know exactly what they need or brace for another search through catalogs and datasheets. This isn’t just another chemical on the list. Choosing the right grade and package shape the outcome, whether it’s for a university project or bulk industry production.
A little confusion still swirls around the grades of 4-Methylpiperidine. Walk down the warehouse aisles or scroll through a supplier’s site and you'll spot “reagent grade,” “technical grade,” and sometimes “pharmaceutical grade.” In real-world work, these grades decide what you’re paying for and how strictly the manufacturer checked for unwanted stuff.
Reagent grade usually pulls the highest price. This one gets used where purity can’t be a guessing game—think life sciences, analytical test kits, and synthesis that demands clear results. Higher purity means the manufacturer checks for identified impurities (often under 1%). For chemists in research, cutting corners here comes with headaches: failed assays, noisy spectra, or worse, hard-to-diagnose contamination.
Technical grade stretches a bit further on the impurity levels. It often fills the tanks of larger-scale chemical plants, agrochemical production, and processing lines that care less about trace leftovers and more about bulk cost. If you’re not working in pharma or high-stakes science, technical grade covers most needs.
Pharmaceutical grade pops up, though less often, when drug intermediates call for strict regulatory approval. Companies have to show paperwork and supplier audits, and any shortcuts risk legal fallout. Here, purity climbs again, but so does scrutiny of every step between factory and end user.
Get an order quote and the next question touches packaging. Sure, it’s easy to imagine a glass bottle for the lab—but in practice, things get strange fast.
Small quantities often show up in amber glass bottles—usually a hundred milliliters or less—sitting snug in foam. These bottles stay popular because they seal tight and the dark glass protects against sunlight. The shipping box sometimes looks better protected than my old wedding china.
Larger amounts often fill aluminum cans, steel drums, or polyethylene containers. Here’s where awkward facts creep in: this stuff doesn’t always smell great, and it has some nasty volatility. In one old lab, a minor leak would leave a fishy, sometimes ammonia-like smell hanging around for hours. Metal containers lock down smell and hold up against rough transport.
Bulk users—say a chemical plant ordering by the barrel—deal with 50-liter or 200-liter drums. Most suppliers only ship in these sizes if a company has proper facilities. Sometimes, specialty packs use lined drums or composite containers—a layer of plastic inside a drum to cut down on contamination.
Those who care about the environment push for returnable packaging or bulk tanks with dedicated transfer lines. In my own work, cleaning up spills from a cracked container felt frustrating; modern packaging avoids this by adding secondary liners or tamper-evidence seals.
4-Methylpiperidine has its hazards, both in the bottle and during transfer. Suppliers now print hazard symbols and handling procedures on each box after pressure from both regulators and field techs. I remember a shipment arriving with only a generic label—it led to hours of double-checking before anyone would crack it open.
Those who cut corners in packaging sometimes pay with lost batches or, worse, workplace incidents. Smart firms invest up front to prevent a disaster, figuring the extra cost beats emergency calls and lost time.
People might think packaging is an afterthought. Anyone who’s cleaned up spilled amines or juggled poorly labeled drums knows better. Choosing the right grade and container isn’t just a paperwork box to tick. It’s how real-world chemists, researchers, and plant managers control quality, costs, and safety—every single day.
| Names | |
| Preferred IUPAC name | 4-Methylpiperidine |
| Other names |
Piperyl methylamine N-Methylpiperidine Tetramethylene-N-methylamine 4-Methyl-1-piperidine |
| Pronunciation | /ˈfɔːrˈmɛθ.ɪl.pɪˈpɛr.ɪˌdiːn/ |
| Identifiers | |
| CAS Number | 626-58-4 |
| Beilstein Reference | **1361171** |
| ChEBI | CHEBI:51340 |
| ChEMBL | CHEMBL15319 |
| ChemSpider | 38447 |
| DrugBank | DB03820 |
| ECHA InfoCard | 100.073.404 |
| EC Number | 203-636-9 |
| Gmelin Reference | 7368 |
| KEGG | C06325 |
| MeSH | D010907 |
| PubChem CID | 7922 |
| RTECS number | EJ8760000 |
| UNII | AX36GX4E6A |
| UN number | UN2389 |
| Properties | |
| Chemical formula | C6H15N |
| Molar mass | 101.19 g/mol |
| Appearance | Colorless liquid |
| Odor | amine-like |
| Density | 0.862 g/mL |
| Solubility in water | Miscible |
| log P | 0.94 |
| Vapor pressure | 3.4 kPa (at 20 °C) |
| Acidity (pKa) | 11.2 |
| Basicity (pKb) | 3.28 |
| Magnetic susceptibility (χ) | -7.45 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.411 |
| Viscosity | 0.773 cP (20°C) |
| Dipole moment | 2.11 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 218.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -30.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4073 kJ·mol⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS06 |
| Pictograms | GHS02,GHS05,GHS06 |
| Signal word | Danger |
| Hazard statements | H225, H302, H314, H332 |
| Precautionary statements | P210, P261, P280, P301+P312, P302+P352, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | 3-3-0 |
| Flash point | -6 °C |
| Autoignition temperature | 280 °C |
| Explosive limits | 1.1–7.4% |
| Lethal dose or concentration | LD50 oral rat 200 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 4-Methylpiperidine: "200 mg/kg (rat, oral) |
| NIOSH | TZ7875000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 1 ppm |
| IDLH (Immediate danger) | 100 ppm |
| Related compounds | |
| Related compounds |
1-Methylpiperidine 2-Methylpiperidine 3-Methylpiperidine Piperidine |