The chemical world always holds a story in every molecule. 4-Methylpiperazin-1-Amine traces its journey back to the mid-20th century, when industrial chemists and academic researchers started investigating piperazine derivatives for their potential in pharmaceuticals and other specialty chemicals. Early syntheses, anchored in basic amination procedures, set the groundwork for further modifications. Metal catalysts and careful pH control emerged as key components, shaping not only yields but also purity. Development over the decades has seen a shift toward greener chemistry, smaller environmental footprints, and a sharper focus on occupational safety. It’s the collective push from both academia and industry, searching for more accessible intermediates and building blocks, that has driven the fine-tuning of 4-Methylpiperazin-1-Amine. As a working chemist, what always stands out is how iterative the process of invention looks: every problem solved brings two more challenges, and the historical legacy of this molecule shows just how persistent the process remains.
4-Methylpiperazin-1-Amine presents as an intermediate that underpins a surprising number of industries. The basic structure offers a stable, customizable backbone where pharmaceutical researchers, crop-protection developers, and even polymer scientists find value. In my experience, this molecule often acts as an anchor for more complex architectures; without it, whole families of compounds would be off-limits. The steady commercial demand underscores its versatility, and every time I look through a specification sheet, the connections between this amine and downstream applications become more apparent. Its wide application only reinforces the importance of getting synthesis, packaging, and purity right.
Labs keep things running smoothly when everyone knows what to expect from their starting materials. 4-Methylpiperazin-1-Amine typically appears as a colorless to slightly yellow liquid or low-melting solid, depending on its purity and storage conditions. Its molecular formula, C5H13N3, signals a compact structure, while a boiling point in the neighborhood of 225-230°C lets chemists plan reactions or distillations with confidence. This compound dissolves well in water and most polar organic solvents, opening up a world of reaction techniques. Density, refractive index, and vapor pressure values line up with expectations for similar heterocyclic amines. Reactivity centers mostly around the primary amine group, which can be a gateway or a liability, depending on how you use it. From personal experience, I've noticed even small impurities in these types of compounds can skew results, lending even more weight to the importance of careful storage and handling.
Quality control is not just for peace of mind—it’s how every researcher or production chemist builds trust in their tools. For 4-Methylpiperazin-1-Amine, detailed certificates of analysis matter. Specification sheets describe purity, often >98%, and lay out acceptable levels for water, heavy metals, and residual solvents. Labels should name the exact batch, reference standards used, and the date of packaging, because nobody wants to guess at shelf stability. In my own projects, relying on incomplete labeling has led to costly reruns and delays further down the pipeline. Regulatory details—compliance with REACH, GHS hazard statements, and transportation classifications—cannot be presented as afterthoughts. Storage requirements (away from oxidation sources or strong acids) also show up on each label. In short, accurate, thorough documentation protects both results and people.
Sourcing 4-Methylpiperazin-1-Amine boils down to a few key synthetic routes. Reductive amination of 4-methylpiperazine with ammonia or other nitrogen sources remains widely used. Lab-scale preparations often rely on hydrogenation reactions or the use of sodium borohydride in alcohol solvents. Continuous-flow setups gain ground in larger settings, where managing heat and pressure delivers sharper yields. Choices in catalysts—ruthenium, palladium, nickel—shape not just yield, but also the downstream environmental impact. Years of bench work have taught me to respect the role minor impurities can play, especially as trace side products end up downstream if purification steps get cut short. Precise temperature and pressure control, paired with vigilant monitoring by GC or LC-MS, create a recipe for reliability. Chemists and chemical engineers never stop looking for greener, safer, and less wasteful synthesis options, an effort seen even in smaller specialty chemical suppliers today.
The reactive amine group offers dozens of pathways for further modification. Coupling the molecule with acid chlorides or isocyanates generates ureas and amides, broadening its applications in ligand libraries and materials science. Alkylation reactions, where the amine picks up longer chains, open opportunities in surfactant chemistry and polymer cross-linking. Oxidation and substitution on the piperazine ring drive much of the molecule’s diversification within pharmaceutical research. In my work, careful pH adjustment during these transformations makes the difference between a respectable yield and a messy side product soup. Multi-step syntheses mean that each conversion must preserve delicate bonds elsewhere in the molecule. Every tweak offers a trade-off between selectivity and throughput, and the best chemists know there are always two ways to do anything—the challenge is finding which one lets you sleep at night.
On the shelf, a single chemical often hides behind several names. 4-Methylpiperazin-1-Amine turns up as N-(4-Methylpiperazin-1-yl)amine, 1-Amino-4-methylpiperazine, and even as some supplier-invented catalog numbers that stick in your mind after years of ordering. These synonyms help coordinate between vendors and research databases, cutting down confusion in multi-lingual, multi-national collaborations. In procurement, getting the labeling right—both common name and CAS number—keeps costly errors off the table and avoids mix-ups with close analogs, which can have radically different properties. Double-checking synonyms saved me from a failed experiment more than once, where a single misplaced methyl group would have set months of lab work back to zero.
Industrial and research settings bring their own risks, especially with amines that can irritate skin, eyes, and lungs. Safety data sheets spell out the risk phrases: gloves and splash-resistant goggles become non-negotiable in any setting where this molecule gets used. Proper ventilation—well beyond a basic fume hood—helps keep airborne concentrations below hazardous limits. In liquid form, 4-Methylpiperazin-1-Amine carries the chance of spills, so spill kits and neutralizing absorbents stay close at hand. Where I’ve worked, regular training and chemical-specific drills shape a safety culture that sticks. Waste streams, especially those containing unreacted amines, need neutralization and careful disposal, never a shortcut into domestic sewage or landfill. Inspections and compliance audits line up with regulatory frameworks set by OSHA and international standards, and the only way I’ve found to stay current is to keep learning from the near-misses you hear about from colleagues.
This molecule finds a home beyond the classic synthetic lab. Pharmaceutical R&D teams deploy it for intermediate steps in the creation of antihistamines, anxiolytics, and enzyme inhibitors, where its flexibility speeds up library synthesis. Agrochemical developers rely on the amine’s reactivity for fast-tracked synthesis of candidate compounds against fungal and insect pests. Polymer science turns to it for chain extenders and cross-linkers, especially in specialty elastomers. Biotech outfits, including custom reagents producers, value the molecule’s straightforward functionality, often coupling it with dyes, peptides, or affinity tags. In my professional orbit, the most creative applications always come from those who see the molecule less as an endpoint, but as a stepping stone for unexpected combinations—leading to advances in both human health and materials technology.
Ongoing research looks at optimizing reaction conditions and designing new derivatives. Competition between universities and private labs over patentable new structures means that even small tweaks—like adding bulkier groups or changing ring saturation—draw attention. Custom synthesis groups build entire workflows around this amine, trying out microwave-assisted techniques or solvent-free reactions. Each attempt at miniaturization and digitization pushes costs down and efficiency up. Researchers publish findings on crystal structures, reactivity trends, and enzyme assays, sharing best practices for the benefit of all. Years in the lab have shown me just how often the best innovations come out of necessity: tight deadlines, budget squeezes, and the pressure to patent. It’s in this environment that new uses for 4-Methylpiperazin-1-Amine continue to appear, whether in bioorthogonal chemistry, material science, or emerging diagnostic technologies.
Toxicological studies on 4-Methylpiperazin-1-Amine show a low to moderate acute toxicity profile, but concerns continue around skin and respiratory sensitization. Long-term data remains limited, with most work focusing on repeated-dose animal models and cell assays. Industrial hygiene professionals watch for cumulative exposure, given the compounding effect of many structurally similar amines. Safety testing, such as the Ames test for mutagenicity, rarely shows strong results for compounds in this family, but a few edge cases underscore the importance of ongoing vigilance. In my own circles, researchers and chemical handlers quickly learn not to underestimate any amine; respect for safety persists, even when repeated tests come back clean. Personal protective equipment and routine health monitoring help close the gap while regulatory agencies finish longer-term studies.
The chemical landscape looks poised for new uses and improved synthesis of 4-Methylpiperazin-1-Amine. As pharmaceutical discovery leans more into automation and AI-guided molecule design, this amine promises even greater value as a customizable scaffold. Greener chemistry trends, including lower-waste catalytic routes and bio-derived starting materials, will likely play bigger roles. Scaling up production to meet new demand, especially in biopharma and performance materials, offers its own challenges—ones that call for inventiveness both in process design and waste management. From my experience, the real excitement lies in what happens outside the lab bench: manufacturers, developers, and end-users working together to push boundaries responsibly. As blogs, journals, and patents continue to flow, this molecule looks set to keep shaping industry and innovation for years to come.
Chemicals with complex names often get ignored in public debates until something goes wrong. Take 4-Methylpiperazin-1-Amine, for example. It rarely grabs headlines on its own. Still, this compound plays a role in a number of important manufacturing processes, especially in the world of pharmaceuticals and specialty chemicals.
4-Methylpiperazin-1-Amine acts as an intermediate, which means chemists use it to make more elaborate molecules. These can include certain medicines targeting diseases or chemical agents that become part of more complicated products. For many companies, finding chemicals that act as solid building blocks can speed up or even make possible the creation of new drugs and treatments. This isn’t an ingredient you find on a store shelf, but it sits in the supply chain of projects that reach consumers in meaningful ways.
For drug creation, pharmaceutical researchers rely on compounds that add specific features to a molecule and help achieve desired effects, like targeting a particular cell or improving effectiveness. Organic chemists try out chains of reactions, picking each step carefully for cost, safety, and reliability. 4-Methylpiperazin-1-Amine serves as a workhorse component along that path, helping scientists reach a final medicine that a doctor might prescribe years later.
Not all uses are benign. Chemical intermediates can pose hazards to health, both for the people producing them and for the communities nearby. 4-Methylpiperazin-1-Amine carries its own list of risks. Breathing its dust or accidentally splashing it can irritate the skin, eyes, or lungs. Factories using this compound pay close attention to training workers, limiting exposure, and setting up emergency routines. Neglect in these areas leads straight to accidents or possible contamination.
Responsible companies don’t just note regulations—they study the full risk chain from lab bench to waste disposal. The public tends to find out about problems after mishaps, but watchdog groups have long pushed for more transparency about what goes on inside chemical plants. Right now, there are differences in oversight between countries with strict government rules and places where enforcement can lag behind fast-moving businesses. Open reporting, community right-to-know initiatives, and unannounced inspections all go a long way, but these measures still face resistance in some regions.
Switching to safer chemicals or reducing the use of hazardous intermediates often makes sense, both for health and the bottom line. Companies have started exploring “greener” alternatives—molecules that break down more easily in the environment or come from renewable sources. Researchers at universities and industry labs have taken this up as a challenge. Newer approaches like continuous flow chemistry aim to keep lower inventories of dangerous compounds onsite, cutting down on the worst risks if something goes wrong.
Everyone from regulators to supply chain managers can push for better monitoring, safer equipment, and investment in safety culture. As someone with firsthand experience working in labs, I know procedures only protect people if everyone on the team believes in them. Occasional box-checking doesn’t work—careful planning, sharing real-world stories of near-misses, and peer accountability pull safety out of the manual and into the daily routine.
4-Methylpiperazin-1-Amine may not show up in news headlines every day, but its role reminds us why we need continued scrutiny over what goes into essential products, and how they’re made. The best chemical innovations come with honest communication, independent oversight, and a drive to solve problems before they make the news.
People working with chemicals like 4-Methylpiperazin-1-Amine aren’t strangers to hazard symbols and warning labels. This compound, like many amines used in synthesis labs or industrial setups, can carry some nasty side effects if it’s handled with no precautions. I’ve had my share of chemical burns and breathing irritation in early lab days, so getting safety right isn’t just ticking boxes; it keeps you healthy and helps everyone go home safe at the end of the day.
This amine comes with well-documented respiratory and skin risks. Direct skin contact can cause burns, itching, or redness. Vapors, especially during mixing or heating, may irritate the nose and lungs. Mistakes with eye exposure get painful fast, and splashes are always possible. I once watched a colleague struggle for days after a splash even though his reaction seemed quick, so taking shortcuts never pays off.
Gloves: Many ordinary lab gloves just don’t cut it. Nitrile or neoprene work better against this substance, and double-gloving with cuffs tucked in means no drips down the wrist.
Eye protection: Safety glasses work for splash protection, but full-face shields add extra safety, especially during transfers or dilutions. I keep a face shield at my bench for anything that could slosh.
Clothing: Lab coats made from chemical-resistant material—and buttoned up the entire way—keep your clothes and skin safer. Closed shoes, long pants, and sometimes chemical aprons help stop accidental contact from spreading if a mess happens.
Respiratory safety: This compound can release some vapor, especially if heated, so work in a fume hood is non-negotiable. A good extraction system means you don’t bring those fumes home in your hair or lungs. I learned the hard way that shortcuts here lead to coughing fits and headaches that last all day.
Storage seems tedious, but anyone who’s witnessed a spill knows why it’s worth the time. 4-Methylpiperazin-1-Amine reacts with acids and oxidizers, so keep it away from these materials. Use sealed, clearly labeled containers. Segregated storage in cool, well-ventilated spots helps keep accidents from turning into emergencies.
Disposal isn’t dumping everything in the sink. Waste must go in labeled, chemical-specific bins. Talking to your site’s waste management team or referencing safety data sheets helps you find safe, legal ways to get rid of extra chemicals. Proper neutralizing—using the right neutralizer for amines—protects the plumbing, the public, and you from unexpected reactions or fines. In my labs, nothing gets poured out unless someone has double-checked the method.
It’s not enough to just wear goggles and gloves. Eye wash stations and safety showers should always stay clear and functional. Running regular safety drills gets everyone ready to respond if something spills, splashes, or gets inhaled. My team runs through these drills every few months, and those rehearsals have saved time and skin more than once.
The last big step comes down to sharing knowledge. Each team member, from newest tech to experienced chemist, stays updated on current procedures and emergency response. Reading the safety data sheet before starting any shift keeps information fresh and prevents confusion during high-pressure moments.
Constant improvement—listening when someone spots a risk, adjusting processes, and investing in better safety gear—keeps accidents from repeating. Watching colleagues learn to speak up about spills or near-misses, I’ve seen firsthand how a culture of safety grows stronger. Safe chemical handling comes down to respect for the risks, solid teamwork, and real preparation every single day.
Chemical structure sits at the core of how a molecule behaves, even when the name sounds a bit intimidating like 4-Methylpiperazin-1-Amine. The arrangement of atoms in a compound holds real value, whether you're mixing chemicals in a pharmacy, designing new drugs, or checking if that mysterious substance is safe to use in a classroom or a lab. Recognizing each bump and bend in a molecule opens up doors to understanding how it reacts with others, how our bodies might break it down, and what kind of effects it might have inside us.
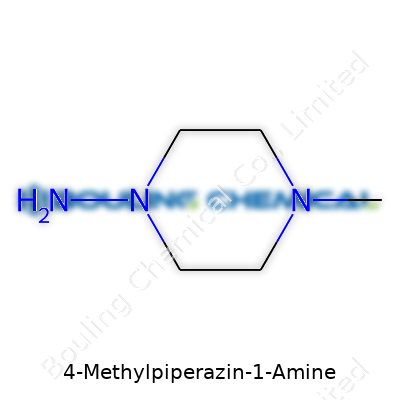
This molecule belongs to piperazine derivatives—a big group in chemistry where the core skeleton is a six-membered ring with two nitrogen atoms. Picture it like a hexagon, not so different from a stop sign, but with nitrogen atoms taking the place of two of the corners. For 4-Methylpiperazin-1-Amine, a methyl group (that’s a carbon attached to three hydrogens, or –CH3) joins at the number 4 spot on the ring, and an extra amine group (–NH2) hangs off the first nitrogen (N1). Each piece adds something: the methyl group makes the molecule bulkier and changes the way it interacts with water and fat, while the amine opens the door for all sorts of chemical reactions and bonds with other molecules.
To get a picture in your head, start with that classic piperazine ring. The six atoms sit in a circle: four are carbons, and two are nitrogens (at the number 1 and 4 positions). From the first nitrogen, imagine a short branch—the amine group. Right across from it, at the fourth carbon, the methyl group sticks out. Chemists draw it as C5H15N3, with an actual structure of (CH3)N(CH2CH2NHCH2CH2), but for regular folks, a ring with two built-in nitrogens plus those side attachments sums it up.
Most people don’t bump into 4-Methylpiperazin-1-Amine at the grocery store, but it pops up in lab supply catalogs and chemical research. The piperazine ring turns up again and again in pharmaceutical chemistry, showing up in medicines for anxiety, allergies, and more. Each tweak—like adding a methyl or amine—shifts its personality. These changes matter because they can make a compound less toxic, more effective, or even allow chemists to target specific sites in the body. In the wrong hands, some amine derivatives can pose risks, so regulations and careful handling keep everything aboveboard.
Learning about molecular structure goes beyond classroom exercises. Safety data sheets give information about hazards, but understanding why something behaves a certain way always helps—especially if you work in science or health. This kind of awareness isn’t just for experts. The more people know about molecular structure, the fewer accidents happen in labs and industry, and the better new products can be designed for everyone’s benefit. Real research, validated sources, and open discussion keep new chemicals moving from theory to practice in a way that benefits all and keeps risks in check.
Walk into any research lab or industrial site, and you’ll spot shelves lined with labeled bottles. There’s a good reason for this careful arrangement: keeping certain chemicals safe protects both people and the environment. 4-Methylpiperazin-1-Amine is not a household name, but those who handle it value clarity, because accidents offer no second chances.
4-Methylpiperazin-1-Amine looks like a typical reagent, but underestimate it and the results may get ugly. This compound reacts with acids and oxidizers, sending off irritating vapors. The toxic effects aren’t merely theoretical—real cases involve eye, skin, and lung burns. The science here lines up with what safety guidelines insist: vigilance isn’t optional.
Climate counts for a lot. A cool, well-ventilated, and dry storage area works best. High humidity or heat cranks up the chances for unwanted reactions or deterioration. Locking 4-Methylpiperazin-1-Amine away from any moisture also means fewer headaches. In my own experience managing smaller research rooms, reliable airflow became the quiet hero, keeping the workspace clear of hazardous fumes without drawing much attention.
Sunlight isn’t just tough on your skin; UV rays break down chemicals like this one over time, sometimes triggering unpredictable results. Opaque or amber containers shield the compound, and storing them away from direct windows further reduces the risk. Thick walls and dark corners in storage rooms offer peace of mind.
Mixing incompatible chemicals leads to trouble fast. Workers have learned—sometimes the hard way—that acids and oxidizers stored nearby pretty much guarantee an emergency. Clear separation remains the simplest, strongest rule. Placing strong acids and bases on opposite shelves, with dedicated spill trays, helps any team sleep a little easier. Labels do more than inform; they save lives. Unambiguous, highly visible chemical labels and updated storage charts keep everyone sharp.
I’ve seen one flimsy cap spoil entire inventories. Spill-proof secondary containment, such as trays or bins, turn small accidents into easier cleanups. Choosing vessels with solid seals cuts down on evaporation or leaks. Nobody wants to scrub up hazardous residue; a few moments spent double-checking closures give back hours in productivity and safety down the line.
Gloves, goggles, and lab coats don’t make you invincible, but they do buy precious seconds during a spill. It helps to keep absorbent materials and neutralizing agents, like sand or vermiculite, readily available within arm’s reach of 4-Methylpiperazin-1-Amine. Quick access to eyewash stations and showers also prevents minor mishaps from becoming emergencies. Training refreshers and regular drills prevent complacency, turning good intentions into automatic, real-world habits.
Regulations aren’t there for decoration. The EPA and OSHA both outline chemical storage standards, expecting organizations to keep airtight logs and routinely inspect chemical stores. Enforcement may feel strict, but incidents involving contamination, injury, or fire attract swift and costly consequences. Adhering to best practices means fewer headaches and less risk of regulatory trouble.
Storing 4-Methylpiperazin-1-Amine doesn’t call for magic tricks or high drama, just practical habits, teamwork, and respect for what the compound can do. As the list of stories about accidents gets longer, each close call reminds us: good storage isn’t a luxury, but a quiet daily investment in safety and trust.
4-Methylpiperazin-1-amine crops up in a range of industrial settings. Ask around in the chemical industry, and you’ll likely hear engineers or lab technicians mention it during talks about making specialty chemicals or pharmaceuticals. Every time I walk into a lab, I check the shelf for new compounds popping up, and this one doesn't ring any safety bells like lead or mercury. Still, it pays to look closer before brushing off its risks for people and the planet.
One sharp fact stands out: 4-Methylpiperazin-1-amine releases strong fumes. Those who’ve spent time in active labs know that the nose is the first line of defense—harsh smells hint at trouble. Even short-term exposure can mean irritation to the eyes, nose, and throat. By direct contact, skin starts to itch or sting, and in some cases, you get redness or even chemical burns if protection slips. Oral intake or breathing high concentrations brings nausea, headache, or dizziness. Over the years, I’ve seen careless handling lead to after-hours emergency calls, especially if workers left gloves off or didn’t use proper ventilation. It always comes back to safety culture and real training, not just reading labels.
Long-term effects take the most attention. Working with similar amines or their relatives like methylamine or ethylenediamine, health data show repeated exposure raises the risk for asthma-like symptoms and allergic reactions. Chronic exposure in enclosed spaces—old labs with poor air flow—often leads to a cough that doesn’t let up and a host of skin problems. Using the right fume hood and disposable gloves, and having good eye protection, helps avoid most of these issues. Still, accidents only need one slip.
Pouring leftovers into a drain or dumping wastelines directly into open water introduces unknowns. Many amines break down in nature, but not every chemical plays by those rules. 4-Methylpiperazin-1-amine hasn't been studied as widely as big-name pollutants, but the EPA classifies related compounds as a risk for aquatic life due to their toxicity. Fish and insects in streams near industrial sites take the hit, and the results don’t show up right away. Frogs with odd limb development or mass die-offs of small invertebrates often lead investigators to run chemical screens, with amines popping up among the suspects.
Real safety starts before opening a bottle. New workers always benefit from hands-on lessons from old pros who know where things go wrong. Always wear chemical splash goggles and nitrile gloves. Before discarding anything down a drain, check safety data sheets and local hazardous waste rules. Many small companies share centralized waste drums to limit accidental releases for these lesser-known amines.
Engineers and managers—people who spend time away from their desks and actually observe what goes on at floor level—build better habits by encouraging reporting of near-misses. In my own career, we learned the hard way that routine air monitoring for these compounds lowers health claims and keeps auditors happy. Across the sector, better labeling of storage containers and double-checking chemical compatibility before mixing waste cuts environmental contamination.
No hidden trick will fix the risk overnight. Sharing lab incidents, pushing for real worker education, and keeping open channels with local regulators lays the groundwork for safe handling. If workers know what 4-Methylpiperazin-1-amine could do to them or the creek outside, they make smarter choices every shift.
| Names | |
| Preferred IUPAC name | 4-methylpiperazin-1-ylamine |
| Other names |
4-Methylpiperazine-1-amine 1-Amino-4-methylpiperazine N-Amino-N-methylpiperazine |
| Pronunciation | /ˈfɔːr ˈmɛθɪl paɪpəˌræzɪn wʌn əˈmiːn/ |
| Identifiers | |
| CAS Number | 29698-76-2 |
| 3D model (JSmol) | `3D model (JSmol)` string for **4-Methylpiperazin-1-amine** (C5H13N3): ``` CCCCN1CCN(CC1)N ``` **Note:** This is the **SMILES** string, which can be used for 3D visualization in JSmol or other chemistry tools. |
| Beilstein Reference | 120926 |
| ChEBI | CHEBI:73056 |
| ChEMBL | CHEMBL127353 |
| ChemSpider | 118759 |
| DrugBank | DB04161 |
| ECHA InfoCard | 03b663a7-60b1-49eb-b88a-d0296e3a43f4 |
| EC Number | 6291-86-9 |
| Gmelin Reference | 107710 |
| KEGG | C14110 |
| MeSH | D060480 |
| PubChem CID | 10309324 |
| RTECS number | GE8400000 |
| UNII | OL676X1UXN |
| UN number | UN3439 |
| CompTox Dashboard (EPA) | `DTXSID80882260` |
| Properties | |
| Chemical formula | C5H13N3 |
| Molar mass | 115.19 g/mol |
| Appearance | White to off-white solid |
| Odor | Amine-like |
| Density | 1.02 g/cm3 |
| Solubility in water | Soluble |
| log P | -0.56 |
| Vapor pressure | 0.0258 mmHg at 25°C |
| Acidity (pKa) | 9.78 |
| Basicity (pKb) | 3.58 |
| Magnetic susceptibility (χ) | -56.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.522 |
| Dipole moment | 2.37 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 277.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4157.8 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes severe skin burns and eye damage, harmful if inhaled |
| GHS labelling | GHS02,GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H314 |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P304+P340, P337+P313 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | 85.0 °C |
| Lethal dose or concentration | LD50 (oral, rat): 773 mg/kg |
| LD50 (median dose) | LD50 (median dose): 870 mg/kg (rat, oral) |
| NIOSH | B3 307 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.05 ppm |
| Related compounds | |
| Related compounds |
Piperazine 1-Methylpiperazine 4-Methylpiperazine N-(4-Methylpiperazin-1-yl)amine N-Methylpiperazine Piperazine-1-carboxamide 1,4-Dimethylpiperazine |