Back in the 1940s and 50s, folks in chemical research started seeking solvents capable of withstanding harsher conditions or supporting ever-more-intricate organic syntheses. 4-Methylmorpholine emerged within this wave of interest, finding a place in the bench chemist’s toolkit. Research papers from the mid-1900s reference the practical need for better intermediates in pharmaceuticals and surfactants, steering companies like DuPont and Dow to patent synthesis techniques. Decades later, production scaled up, especially as polyurethane chemistry took off. Today’s manufacturing facilities rely on efficient, environmentally responsible processes, largely because older syntheses suffered from poor yields and harsh conditions.
4-Methylmorpholine stands out in laboratories and industry for its versatility. The compound shows up in processes for making drugs, coatings, flocculants, and polymers. Its structure, a morpholine ring with a single methyl group, gives it select properties chemists pursue: solid miscibility with water and many organics, and enough stability for rough-and-tumble industrial use. You'll see barrels labeled with major brand names, each pitching purity or handling advantages, but underneath the label it's the same clear, amine-scented liquid.
Pour a sample of 4-Methylmorpholine, and you get a colorless liquid carrying a distinct ammonia-like aroma. This liquid boils at around 116°C, so it's manageable under standard lab or plant conditions. Its density hovers near 0.92 g/cm³, lighter than water but not so volatile you lose the sample in a few minutes. The flash point sits at about 21°C, which calls for safe, controlled storage. As a base, it's strong enough to deprotonate weak acids, and its miscibility with water gives users wiggle room to tweak solvents and catch subtle changes in solution. The methyl group changes its electron distribution compared to regular morpholine, so it sometimes reacts differently in a synthetic route.
You can pick 4-Methylmorpholine up in technical grades that promise 99% or better purity. Reputable distributors back up their label with batch-specific assay data. Advanced quality control not only confirms content but includes water level, color, and the presence of morpholine or nitrosamines—byproducts that careful technicians want to keep in check due to potential toxicity. Commercial drums often come with full GHS labeling, pictograms for flammability, and a detailed SDS that helps anyone in the plant access safety details in a pinch.
The classic route for 4-Methylmorpholine involves cyclizing diethanolamine with formaldehyde plus methylating agents such as methanol under pressure, all in the presence of hydrogen and a nickel catalyst. Over the years, technical improvements have trimmed down waste and shrunk carbon footprints by using better catalysts and reactors, which means today’s production sheds less off-gas and runs at higher yields. Some companies use continuous flow methods to keep volumes up and batch time down, while others stick to time-tested, slower syntheses where purity matters most.
As a secondary amine, 4-Methylmorpholine jumps into acylation, alkylation, and oxidation reactions. Its role as a base makes it an efficient scavenger in acid-catalyzed steps during pharmaceutical runs. Chemists sometimes substitute it for more hazardous amines when adjusting pH or as a catalyst in urethane systems, given its slightly less aggressive qualities. Ethoxylation, sulfonation, and even N-oxide formation push the compound into new chemical spaces, each unlocking properties tailored to performance additives in coatings or cleaning products. With its stable ring, it can take some rough treatment in reactors, unlike more fragile amine choices.
The material also shows up under names like N-Methylmorpholine or 4-Methyl-1-oxa-4-azacyclohexane. In catalogs, brands may label it “Methylmorpholine”, “NMM” or assign internal product codes. International standards like CAS numbers nail down its identity despite the name swaps. Trade literature often highlights the same batch-to-batch product, whether sold for synthesis or as a blend component.
Everyone handling 4-Methylmorpholine needs to respect its hazards. The liquid can irritate eyes, skin, and the respiratory tract, so basic lab gloves and goggles aren’t optional. In big plants, full-face shields get paired with chemical splash suits and proper ventilation, since vapors can build up and cause headaches or worse. The material’s low flash point puts it in the category of flammable liquids, and every warehouse should store it away from oxidizing agents or open flames. Disposal practices now follow strict environmental guidelines, preventing contamination. Training by EHS managers and routine air monitoring add another layer of real-world protection.
4-Methylmorpholine makes itself useful in many areas. In the world of polyurethane chemistry, it serves as a catalyst pushing reactions towards better foam quality. Paint and coatings makers turn to it for its miscibility and buffering ability. Paper treatment, water purification, and pharmaceuticals (as a building block for drugs treating infections or blood pressure) all tap into its strengths. In more niche research, scientists use it when making surfactants or specialized polymers that need unique amine functionality. Its ability to modify chemical environments without introducing heavy metals or salts gives it legs in green chemistry initiatives, which industry now follows closer than ever.
Research teams continue to dig into ways to squeeze more performance out of 4-Methylmorpholine. Companies investigate alternate feedstocks derived from renewable resources to lower carbon emissions in manufacturing. Academic labs test catalysts that shorten reaction times, lower energy use, or give rise to byproducts easier to recycle or treat. In pharmaceutical development, chemists hunt for derivative molecules offering higher selectivity or reduced side effects. One hot area is creating N-oxides from 4-Methylmorpholine, which can boost oxidation reactions or serve as mild bleach additives. Partnerships between environmental scientists and chemical manufacturers study biodegradation, hoping to minimize long-term impact.
Toxicologists keep a close eye on 4-Methylmorpholine’s effects on workers and the environment. Inhalation studies flagged low-to-moderate acute toxicity, but chronic effects remain under scrutiny. The compound has passed many genotoxicity tests, yet long-term ecological risk studies grow in number as society pushes for safer industry. Researchers now monitor blood acetylcholinesterase levels among frequent handlers, and some animal studies show reversible effects on nervous tissue at high doses. Wastewater treatment plants set new guidelines, limiting how much morpholine-type amines can enter sewer systems.
Looking ahead, 4-Methylmorpholine faces the push for safer, more sustainable chemical ingredients. Demand for cleaner polyurethane foam means research dollars continue to flow into catalyst optimization. Green chemistry may deliver a process converting ethanol-based diols to morpholine scaffolds via engineered microbes, sidestepping petrochemical feedstocks entirely. Growth in clean energy, medical device coatings, and biodegradable surfactants could all stretch demand further. Policy changes at the government level spur innovation in waste management and worker safety, so manufacturers evolve plant practices to keep pace with regulations. Every few months, new patents emerge for derivatives showing up in medical imaging, targeted therapies, or hyper-efficient batteries, each building on the backbone of this simple, six-membered ring.
Many of the products we use every day rely on simple chemicals working behind the scenes. One of those is 4-Methylmorpholine, a colorless liquid with a mild ammoniacal smell. Anyone who has set foot in a laboratory or worked in manufacturing might recognize it for its versatility. In my time working with chemical suppliers, 4-Methylmorpholine kept showing up on packing slips headed to factories making everything from furniture to medicines.
You will likely find 4-Methylmorpholine in facilities where polyurethane foam forms the backbone of production. The chemical acts as a catalyst, nudging the reaction between polyols and isocyanates, which leads to the foams used for mattresses, insulation, and car seats. Factories rely on it because it helps control how fast the foam rises, firms up, or softens. Quality control teams pay attention to the catalyst mix, knowing small changes mess with the end product’s shape and function. Last year, I spoke with a production manager from a major foam plant near my town. He explained how swapping out cheaper catalysts never worked as smoothly as sticking with the blend featuring 4-Methylmorpholine. That blend made the process more consistent, which meant less waste and better yields.
Chemists use 4-Methylmorpholine as a solvent because it dissolves many reactants and doesn’t react with as many chemicals as harsher solvents can. In the pharmaceutical world, manufacturers turn to this chemical for specific reactions, such as preparing drug intermediates. In my experience, choosing a good solvent saves time and money, especially when researchers work under tight deadlines. One reason labs keep 4-Methylmorpholine on hand is its balance of solvency and manageable volatility - it doesn’t evaporate too quickly but still keeps everything flowing. It’s also less likely than other amines to give off strong odors that clear out a lab.
Companies often use 4-Methylmorpholine to make other chemicals. Manufacturers produce morpholine derivatives—ingredients found in corrosion inhibitors, rubber chemicals, and textile agents—starting with this compound. Textile finishers might add these derivatives to keep fabrics from absorbing too much water, making raincoats and gym bags last longer. I once had a client in textile finishing who switched to a morpholine derivative for their fabric treatment line; the improvement in durability meant happier customers and fewer returns.
Nothing about handling 4-Methylmorpholine comes risk-free. The vapor, if allowed to linger, irritates eyes and breathing passages, so proper ventilation means everything. Workers suit up with gloves and goggles because even a splash on the skin can sting. Regulatory bodies such as OSHA and the European Chemicals Agency push for labeling and careful storage, a step in the right direction. Back in college, I remember a friend getting a mild chemical burn from not double-checking their gloves adding 4-Methylmorpholine to a reaction flask. It drove home how important respect for industrial chemicals remains.
The environmental picture isn’t perfect. If released outside standard disposal methods, this chemical could harm aquatic life. Industry leaders focus on closed-loop systems and spill prevention, aiming to hold companies to higher safety standards. Some suppliers now audit their disposal and transportation processes, not just to follow rules, but to reassure communities living near their facilities that safety isn’t optional.
While alternatives pop up in research, companies lean on 4-Methylmorpholine for its track record. That means keeping up with best practices matters. Those working with it owe their coworkers and neighbors a duty to stay vigilant, monitor air quality, and share information about new safety options.
Talking about chemicals like 4-Methylmorpholine can make some people uneasy, especially if you have never worked in a lab or a chemical factory. This compound pops up in several industrial processes, most often as a solvent or intermediate for making things like coatings, pharmaceuticals, or even rubber. If you walk into a facility handling this substance, it’s not sitting out where anyone can get close to it for fun—it stays in controlled environments.
Breathe in a strong vapor of 4-Methylmorpholine, and you’ll notice. It can irritate the nose, throat, and lungs. People complain about headaches, dizziness, or even nausea after exposure. Get it on your skin and you’ll likely feel irritation, maybe even burning after enough contact. Eyes exposed to its vapors water and sting, something you won’t soon forget. Data from the U.S. National Library of Medicine and the European Chemicals Agency back up these experiences. These agencies highlight its toxic nature in high concentrations and consider it a risk for those not wearing protective equipment.
Accidentally swallowing any chemical like this is a trip to the emergency room waiting to happen. Animal studies show that moderate doses damage kidneys and liver. Workplace safety sheets call out possible risk of lasting health problems when not handled carefully. Over several months or years, repeated exposure ups the risk of chronic headaches, drowsiness, and skin conditions.
Protective gloves, lab coats, eye protection, and serious ventilation—these aren’t just guidelines from government documents. In my years working in a chemistry lab, safety routines ruled every move. Training wasn’t a formality. The smell of amine compounds like 4-Methylmorpholine throws up an automatic alert in your head. Vent hoods stay on, gloves come off before touching your face or phone, and everyone keeps an extra set of goggles handy.
Emergency eyewash stations don’t sit in labs just for appearance. I’ve seen coworkers splash a chemical on their sleeves or wrists, rush to rinse, and then get checked out just in case. No one shrugs off a chemical burn, not with 4-Methylmorpholine around.
Safety standards from OSHA and the European Agency for Safety and Health at Work expect workers to limit exposure to 4-Methylmorpholine, monitor air quality, and keep cleanup procedures ready. Fines and inspections fire up quickly if companies cut corners. Factories near neighborhoods must follow spill reporting rules and maintain storage tanks under lock and monitoring. Studies of communities next to chemical plants sometimes spot odor complaints or short-term symptoms, but large spills or heavy airborne releases remain rare thanks to tight controls.
Respect for chemicals like this doesn’t grow from paranoia—it grows from experience. Frequent air checks, careful lab habits, and solid emergency plans all play a real part in health and safety. For folks handling these materials every day, small changes like switching gloves mid-shift or updating training after minor spills have a lasting impact. Local clinics and poison control centers make a difference by educating workers and neighbors, helping the warnings stick even after the training ends. The safest workplaces share one trait—everyone talks openly about what could go wrong and how to fix it fast.
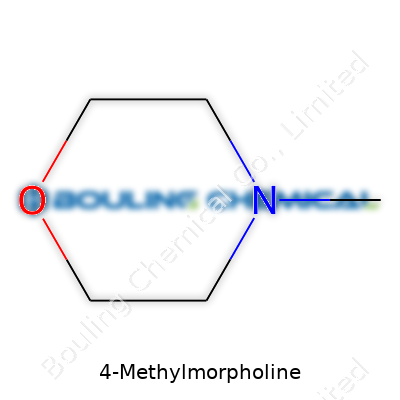
Plenty of folks work with chemicals every day and never stop to think about what goes into their products. 4-Methylmorpholine might not be a household name, but in labs and factories, you’ll see this compound show up on more labels and safety documents than one might expect. Its chemical formula, C5H11NO, tells you straightaway what’s in the bottle: five carbon atoms, eleven hydrogens, a nitrogen, and one oxygen atom.
You can spot the “morpholine” base—a six-membered ring containing both an oxygen and a nitrogen. By swapping in a methyl group at the fourth position, chemists give the molecule unique properties that morpholine doesn’t have alone. That small tweak makes a big difference in how factories use it and how folks need to handle it.
Understanding the formula isn’t busywork. It sits at the core of safety, environmental responsibility, and product integrity. Let’s say I’m mixing up a batch of polyurethane foam. The formula tells me what reactivity to expect. 4-Methylmorpholine often speeds up curing, helping pieces set without waiting around. Miss the formula, and you might blend it with other amines, never realizing one does the job faster, or in a slightly different way that throws off fire resistance or durability.
It’s not just chemists who care about structure. Regulatory bodies and environmental safety officers watch it closely. C5H11NO breaks down into predictable byproducts—they want to track whether emissions or waste streams contain things that could harm water supplies or air quality. Being able to identify, monitor, and control these substances starts with knowing the molecular formula.
Years of working with solvents and reagents taught me that nothing beats attention to detail. The smallest shift—swapping one group in a molecule—can trigger unpredictable outcomes. If you’re training new staff, you don’t just hand them a bottle and say “pour this in.” You teach them how formula shapes hazard. 4-Methylmorpholine brings moderate toxicity—exposure can irritate the skin and eyes, and breathing vapors at high concentration isn’t safe. I’ve seen shops get citations for improper handling, all because someone overlooked the data sheet.
Sustainability teams ask tough questions. Can we tweak formulas to cut out petroleum inputs or lower toxicity? The formula locks in certain pathways, but smart chemists keep pushing at the edges: tweaking conditions to reduce hazardous waste, improving ventilation, or shifting toward alternatives when they fit the bill. In my own experience, switching to less persistent chemicals often required running pilot tests just to match the productivity and ease of use that 4-Methylmorpholine supplied.
These chemicals don’t just teach lessons about molecular structure; they show how small details can save time, money, and trouble. C5H11NO may just look like a string of numbers and letters, but that’s the foundation for every best practice in the industry. By understanding what’s in the tank, labs build better products, waste less, and protect people and the environment. That matters wherever chemistry touches daily life—which turns out to be just about everywhere.
4-Methylmorpholine isn’t a household name, but anyone who’s worked in a synthetic chemistry lab recognizes its sharp, amine-like odor right away. Used as a building block in dyes, pharmaceuticals, and even some coatings, it has its place in industry for sure. But with that usefulness comes risk: this liquid easily irritates skin and eyes, and can give off flammable vapors above room temperature. From personal experience, you never want to learn those hazards the hard way.
Up on the shelf next to common solvents like ether or acetone, things can get dicey fast. 4-Methylmorpholine’s flash point sits around 33°C. That puts it in the group of chemicals that don’t need summer heat to throw off enough vapor for ignition. Flammable vapors in a confined space spell disaster—think of a spark from static, or a forgotten hotplate. So forget about storing it near heat sources or in direct sunlight.
The best place for this liquid is inside a flammable safety cabinet. If you’ve ever seen those yellow metal lockers in a lab or workshop, you know what I mean. Solid metal construction and self-closing doors don’t just look good for an inspection—they’re what stand between a routine day and an emergency. Plus, built-in ventilation helps keep vapors from building up, which keeps everyone safer.
Chemicals rarely cause trouble on their own. The real problems show up when bottles mix, whether from spills, leaks, or simple carelessness. 4-Methylmorpholine reacts with acids, so you’ll want a separate spot from nitric, sulfuric, or hydrochloric. Piling acids and organics side by side on a crowded shelf has led to more than a few workplace evacuations. In any seasoned lab, marked shelves for acids, bases, and organics keep those headaches to a minimum.
I’ve seen a bottle of an amine like this leak onto acid-stained gloves in a pile. The smell alone could clear a room, but you’re risking more than a bad odor with uncontrolled mixing—corrosive gases or even fires can result if safety slips.
A simple screw-cap bottle might do the trick for water, but 4-Methylmorpholine deserves better. Only use containers made from materials that resist amine attack: high-density polyethylene or amber glass both work well. Make sure every bottle carries a clear label, with name, hazard signs, and date received. When a few nearly identical bottles line up, faded or missing labels quickly confuse even experienced hands. Anyone who’s inherited an old storeroom full of mystery chemicals knows the headaches—and the risks—of unidentified containers.
Complacency sneaks in most often on quiet days. Keep keys to the flammables cabinet with trained staff, not just anyone who wanders by. If you train everyone once and expect perfect recall, you’re in for a surprise. Routine walk-throughs, daily checks for leaks or spills, and regular hazard reviews all help keep folks sharp. I’ve had a supervisor spot a tiny cap left loose—one simple catch stops a cascade of trouble.
The best practices aren’t just fancy regulatory speak—they keep people safe, avoid costly accidents, and make every shift smoother. Proper storage is about real vigilance. If a chemical gives off fumes or reacts with everyday acids, treat it with the same respect you’d expect in someone else’s lab. A little preparation goes further than you think: proper cabinets, labels, and teamwork mean everyone goes home safe at the end of the day.
Working with chemicals like 4-Methylmorpholine never feels routine, no matter how experienced you get. This colorless, flammable liquid has a sharp, fishy smell that lingers, and it can irritate skin, eyes, and the respiratory tract pretty quickly. There’s no room for shortcuts or guesswork here. The risks aren’t just about personal exposure; spills and evaporation put coworkers, waste handlers, and the environment on the line, too.
4-Methylmorpholine stings on contact. Standard gloves—nitrile or neoprene—form the first barrier. Some folks reach for latex, but that material breaks down fast with organic amines. Chemical splash goggles block eye irritation, and a lab coat or apron handles most splatter. Labs or plants storing big drums should always have eyewash stations and safety showers close by. I’ve watched simple splashes turn into ER visits for new team members who underestimated just how fast this solvent reacts.
Neglecting ventilation turns air quality risky fast. Good fume hoods pull fumes away before they get inhaled or spread through the workspace. Relying on open windows won’t cut it; point extraction at the bench or mechanical systems offer actual control. Facilities planning for regular use of 4-Methylmorpholine need proper ducted enclosures—and frequent checks of filter systems. Breathing in vapors brings headaches, dizziness, and far worse consequences overtime due to chronic exposure.
Labeling this chemical “flammable” isn’t just for paperwork. This solvent has a pretty low flash point, so ignition sources—sparks, static, and open flames—have to stay away. I’ve seen fire start from a hot plate left unattended just *once*, and that drilled the lesson home for good. Store containers in flammable liquid cabinets, and keep only what’s needed for immediate work on the bench. Stacking barrels in a corridor or backroom means trouble when a leak turns up.
A spill turns worry into immediate action. Fast containment using absorbent pads, then decontaminating the area with water and detergent, brings risk back under control. Handling clean-up by hand needs extra care; splash-resistant gloves, thick enough to hold up through scrubbing, make a difference. Never let contaminated cleanup waste join the regular trash. Dedicated disposal follows local hazmat rules, and every team needs to know those steps cold.
Labels on primary and secondary containers keep confusion out of the equation. Practiced, clear training—both initial and refresher—is what keeps safety habits strong. It’s hard to replace muscle-memory for emergency eyewash use or evacuation drills once an accident hits. Data sheets and signage need to stick around, not gather dust in a drawer. Experience doesn’t excuse skipping the small steps; mistakes feel real for everyone they catch off-guard.
Surfaces where 4-Methylmorpholine sits should resist corrosion and absorb spills without damage. Stainless steel benchtops wipe down easily without lingering residue. Loose plastic liners or torn mats trap the chemical underneath, where it spreads or evaporates unnoticed. Regular inspections and cleaning routines spot trouble early. A sticky floor, a strange smell—these details signal it’s time to halt operations and reset the workspace.
Industry leaders and lab supervisors keep scouting safer alternatives and engineering solutions. Substituting less toxic solvents for specific processes sometimes pays off, without sacrificing quality results. Adoption of automated pipetting or liquid-handling systems can shrink exposure opportunities. Peer review of procedures and ongoing risk assessments spark discussions that bolster both personal safety and environmental health.
| Names | |
| Preferred IUPAC name | 4-Methylmorpholine |
| Other names |
N-Methylmorpholine 4-Methyl-1-oxazine N-Methyl-tetrahydro-4-pyranol |
| Pronunciation | /ˌfɔːrˌmɛθ.ɪlˈmɔː.fə.liːn/ |
| Identifiers | |
| CAS Number | 109-02-4 |
| Beilstein Reference | 1091103 |
| ChEBI | CHEBI:35785 |
| ChEMBL | CHEMBL143055 |
| ChemSpider | 9573 |
| DrugBank | DB04126 |
| ECHA InfoCard | 100.017.850 |
| EC Number | 203-678-3 |
| Gmelin Reference | 8280 |
| KEGG | C14385 |
| MeSH | D008943 |
| PubChem CID | 7785 |
| RTECS number | QD6475000 |
| UNII | 1L8W1455HY |
| UN number | 2810 |
| Properties | |
| Chemical formula | C5H11NO |
| Molar mass | 101.16 g/mol |
| Appearance | Colorless liquid |
| Odor | Ammonia-like |
| Density | 0.922 g/mL at 25 °C (lit.) |
| Solubility in water | miscible |
| log P | 0.44 |
| Vapor pressure | 7.2 kPa (at 20 °C) |
| Acidity (pKa) | 8.4 |
| Basicity (pKb) | pKb = 4.92 |
| Magnetic susceptibility (χ) | -41.5e-6 cm³/mol |
| Refractive index (nD) | 1.422 |
| Viscosity | 1.74 mPa·s (20 °C) |
| Dipole moment | 2.0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 273.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -267.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4133.9 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02, GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H312, H314, H332 |
| Precautionary statements | Precautionary statements of 4-Methylmorpholine: "P261, P264, P271, P280, P301+P312, P304+P340, P305+P351+P338, P312, P330, P337+P313, P405, P501 |
| NFPA 704 (fire diamond) | 2-3-1 |
| Flash point | 64 °C |
| Autoignition temperature | 187 °C |
| Explosive limits | 1.8% - 11.2% |
| Lethal dose or concentration | LD50 oral rat 930 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 2,200 mg/kg |
| NIOSH | WT4825000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 4-Methylmorpholine: 20 ppm (parts per million) |
| REL (Recommended) | 4 mg/m³ |
| IDLH (Immediate danger) | 100 ppm |
| Related compounds | |
| Related compounds |
N-Methylmorpholine 2-Methylmorpholine Morpholine 4-Ethylmorpholine |