The rise of 4-Methylmorpholine 4-Oxide, Monohydrate, often shortened to NMMO monohydrate, speaks volumes about the directions research took in the twentieth century. Originally tucked away in specialty labs, NMMO appeared in chemical literature as experts searched for cleaner routes to process plant fibers. Early patents around the 1930s only hinted at its later value. Once the textile world caught wind that this solvent could dissolve cellulose, innovation snowballed. The fiber spinning sector adopted the Lyocell process with NMMO at its core. Massive companies poured resources into scale-up, researchers tweaked the hydration levels, while chemical engineers tackled heat management. In factories, labs, and regulatory agencies, work on this compound turned into a masterclass on material science’s reach and the bumps that always show up when chemistry leaves the bench for the warehouse.
NMMO monohydrate carves out a niche where strong solvents matter but harsh chemicals won’t cut it. It looks like a pale yellow or colorless crystalline substance, melting a little above room temperature. Solutions handle cellulose pulp without the noxious effects that come from traditional acids. Folks in textiles value this because you get pure fiber and fewer byproducts, supporting the steady march toward “greener” industry processes. Whether shipped in drums to major fiber plants or packed in small samples for research, it remains consistent: consistent melt point, consistent effect on cellulose, consistent headaches if you ignore moisture control.
Ask any chemist who’s handled NMMO, and they’ll list a handful of quirks that need respect. It melts near 70°C, picks up water from the air in a hurry, and, as anyone who’s pushed Lyocell technology knows, can decompose, releasing hazardous fumes if things get too hot. The monohydrate carries one water molecule for each molecule of NMMO—drop more moisture, the properties start shifting. With the right setup, NMMO sits harmless, but it can catalyze runaway reactions if folks get careless. It blends hydrogen-bonding with powerful solvating ability, which opens up not just fibers, but entire markets.
Strict purity standards keep industrial NMMO in check: folks running Lyocell-grade stock demand over 96% purity with low heavy metal content. Most suppliers list water content in the 13%-15% range for monohydrate forms, since small shifts throw off fiber spinning. Storage specs look strict because the compound absorbs water and degrades above certain temperatures. Packages must carry hazard symbols for irritants and oxidizers; labeling codes like CAS 13060-54-6 track inventory, and material safety data sheets warn of the fire and fume risks from decomposition. The combination of solvent strength and sensitivity narrows the line between safe application and emergency protocols.
Commercial production involves oxidizing 4-methylmorpholine using hydrogen peroxide, often in tightly controlled reactors to avoid runaway exothermic reactions. Process control stands front and center—chemists add hydrogen peroxide to cooled, stirred solutions for hours, then fine-tune the water content during isolation. Even small errors can cause a lot of product to fail spec and turn disposal into an expensive chore. I’ve seen startup labs fumble on the water content adjustment step, losing product or having to salvage odd hydrates. Producers learned early that timing, temperature ramps, and scrupulously dry containers define whether they’ll pull out white, usable crystals or start over with what looks like a mess.
NMMO’s fame comes from its dissolving power, not because it sits inert. In fiber work, it breaks down cellulose chains into spinnable solutions, then releases new fibers without need for harsh acid regeneration. It acts as a mild oxidizer in some organic reactions, swapping its oxygen under precise conditions. Tweaking the hydration or mixing in co-solvents expands its reach, from biopolymer processing to catalysis in specialty syntheses. Anyone who’s worked with NMMO in a synthetic lab learns that going outside the comfort zone risks side reactions: decomposition kicks up formaldehyde and methylmorpholine vapors, especially under heat or improper pH, limiting casual modifications.
Researchers and manufacturers stick to a list of names—N-methylmorpholine N-oxide, NMMO monohydrate, and by trade names like Amplifin®. Chemical catalogs pile these labels together so procurement runs smoothly globally. Paperwork trails use these synonyms to confirm specs match research citations, purchasing agreements, and regulatory filings. In older patents, the ingredient list reads slightly differently, a sign of how evolving language tracks broader industrial acceptance.
Despite its utility, NMMO monohydrate demands close attention for worker health and facility safety. It draws moisture from the air, making it slippery to handle. Fire risk spikes above 120°C, particularly because decomposition releases toxic fumes. I’ve watched plant managers run air exchangers day and night when heating vessels to keep levels safe. Workers suit up with gloves, goggles, and sometimes respirators, depending on the setup. Eye protection and localized exhaust systems show up in every safety audit. Spill kits for NMMO combine absorbent material with neutralizers, standing by for leaks. Global regulations push for constant review of exposure limits and waste procedures.
No single use takes up as much NMMO as the Lyocell fiber industry. Here, massive tanks filled with NMMO dissolve wood pulp into honey-like liquid before spinning it into threads for textiles. NMMO’s strength sits in its selective solvency, pulling apart cellulose chains without unwanted byproducts, letting finished threads find their way into everything from fashion to medical swabs. Some enzymatic research and specialized organic reactions pick NMMO as a co-catalyst or mild oxidizing agent, but cost and safety rules fence off small-scale users from big adoption. A handful of green chemists eye it for new biopolymer solvents, yet most budgets don’t outweigh the infrastructure investment Lyocell already swallowed up.
Research teams continue probing NMMO for next-gen fiber processing, but also for so-called “difficult-to-dissolve” biopolymers, hemicelluloses, and specialty cellulose derivatives. I’ve watched graduate students run months of experiments just optimizing water content for spinnable dope or hunting for stabilizers that block runaway degradation. Some studies eye modifications using ionic liquids or better process control for improved recyclability and energy use. Academic labs publish new reviews almost every quarter, wrapping up everything from spectroscopic studies to pilot-plant trial results. Industrial R&D keeps lining up methods that try cutting back water or power use, balancing production efficiency with worker health targets.
Most animal studies flag NMMO as not overtly carcinogenic but point to tissue irritation, particularly in lungs and eyes. Acute exposure delivers burning sensations, showing just how fast the compound can go from useful to hazardous. Investigations with fish and plants note risk around effluent from large-scale fiber plants, with aquatic toxicity drawing more attention from regional agencies. Process development folks face stricter wastewater targets because NMMO’s long tail of decomposition products hasn’t been mapped completely. Long-term exposure studies chase any subtle neural or developmental shifts, laying the groundwork for the next round of workplace guidelines.
There’s plenty of conversation among chemical engineers about where NMMO goes from here. Lyocell’s popularity keeps driving supply and process improvements, especially with rising demands for recycled and less wasteful fibers. Researchers test NMMO for custom polymers or as a solvent in more sustainable pharmaceutical manufacturing. Environmental concerns tighten the leash on disposal and emissions, pushing everyone to recapture and recycle solvent streams. Swapping to alternative oxidation chemistries, or finding stabilizers resisting degradation, keeps running through patent filings and conference talks. The compound holds its ground as a go-to for cellulose transformation, though shifting regulations and new green chemistry tools may one day disrupt the status quo just as NMMO did to old acid-based methods.
People love natural fibers, and demand for eco-friendly textiles keeps growing. One compound, 4-Methylmorpholine 4-Oxide, Monohydrate, often called NMMO, finds itself right in the thick of fiber innovation. Chemists use NMMO to dissolve cellulose, which comes from wood pulp and plants. This gives us a way to spin cellulose into yarn without the harsh chemicals linked to older rayon processes. As someone who’s spent time hands-on with sustainable materials, the value this brings to clothing and textile industries really stands out.
Ever felt a tencel shirt? That smooth, gentle touch comes from a fiber made possible by NMMO. This chemistry lets manufacturers break down plant cellulose and reshape it into strong, absorbent threads. What sets this apart is the closed-loop process, basically a production cycle where water and the solvent get recycled, cutting down on waste. Compare this with viscose manufacturing: viscose relies on carbon disulfide, a toxic chemical linked to air pollution and worker health issues. With NMMO-driven lyocell production, nearly 99% of the solvent sees recovery and reuse, so toxic emissions drop dramatically. It’s a big step up for both the environment and for worker safety.
Not everything about NMMO succeeds without obstacles. It reacts with oxygen and heat, so production needs careful attention to temperature controls. NMMO can break down explosively if mishandled. As an operator or someone managing large-scale manufacturing, that’s a real concern. Some companies invest in better safety controls, such as airtight reactors and steady cooling systems. That said, improvement is always possible. More research into stabilizers and alternative process designs could help keep things both efficient and safer for people on the floor.
The chemistry world uses NMMO for more than just spinning fibers. In labs, scientists use it to break down cellulose so they can study its inner workings or create new compounds. Some researchers work on modifying papers and membranes for filtration or medical use with NMMO. Its power to dissolve tough cellulose gives material scientists more room for experimentation. This opens the door to high-performance fabrics and specialty papers, and can also create avenues for biodegradable plastics, which could help ease part of the plastic waste crisis piling up around us.
Demand for sustainable consumer goods is not going away. If anything, we’ll need more green chemistry solutions—ones that cut down pollution, recover resources, and enable new biomaterials. NMMO gives us an option to work responsibly with plant-based cellulose. For any company facing stricter regulations and a rising call from the public for cleaner production, looking towards technologies built on solvents like NMMO means taking real steps toward lower impact manufacturing.
People and companies who use 4-Methylmorpholine 4-Oxide, Monohydrate must keep safety and stewardship front and center. That means investing in regular training, upgrading equipment, and taking honest stock of risks. There are ways to improve recovery rates and keep the chemical out of the environment. I’ve seen first-hand how a skilled operator can keep things both safe and efficient. No one person or single product will solve the sustainability puzzle, but NMMO stands out as a practical tool for those who care about greener industry and safer workplaces.
4-Methylmorpholine 4-oxide, monohydrate—better known in labs as NMMO—often pops up in the world of textile and cellulose processing. This chemical serves as a powerful solvent, especially in the production of Lyocell fibers—think about those soft, sustainable fabrics marketed as eco-friendly. With all its promise in green manufacturing, people still need to pay close attention to the darker side: its safety and toxicity.
NMMO’s major hazard comes from its ability to irritate skin and eyes. Experience in laboratory settings and chemical factories has shown that accidental splashes can cause redness, discomfort, and even blistering in severe cases. If the powder or solution becomes airborne, inhalation might cause coughing, throat irritation, or—even less obviously—headaches and dizziness.
Industry guidelines advise using gloves and goggles, not just because of some blanket rule, but because workers who have dumped NMMO on bare skin often report painful rashes. NMMO readily absorbs water and draws it from tissue, amplifying irritation. Data from the European Chemicals Agency marks it as an irritant, but—despite various rumors—it isn’t classified as outright carcinogenic or mutagenic.
No long-term human studies exist that track NMMO exposure over decades. Still, the National Institute for Occupational Safety and Health references animal studies hinting at liver and kidney stress after repeated high-dose exposure. This remains a concern for workers handling the chemical year after year in places without strong ventilation or routine health checks. Occupational asthma and dermatitis sometimes show up, highlighting the need for real monitoring, not just dust masks tossed in a drawer.
Disposal has also raised eyebrows. NMMO runoff—left untreated—can stress aquatic life. Reports describe fish and invertebrates avoiding sections of water downstream from textile production sites in countries with lax chemical laws. This isn’t just hypothetical: improper containment has killed fish and disrupted entire river ecosystems.
NMMO gets tagged as “less toxic” than classic cellulose solvents like carbon disulfide or concentrated sulfuric acid. There’s truth here. Fewer deaths and hospitalizations show up in the journals, and its popularity in the Lyocell process reflects a step forward from the old hazardous ways. Still, “less toxic” shouldn’t mean careless. Sloppy storage or a clogged fume hood can easily turn NMMO into an emergency.
Regulators in the United States and European Union keep NMMO on watchlists for workplace safety. Factories using it must submit exposure data, injuries, and waste management plans. Random inspections and stricter personal protective equipment rules remain common, especially in countries with high environmental standards. I’ve met workers who still recall older textile plants where fumes hung in the air and gloves were rare—many now deal with chronic breathing problems or skin issues.
Improved engineering controls help the most—closed-loop systems that trap vapors, automated feeders that reduce direct contact, and continuous training for new and veteran staff. Mandatory health surveillance and environmental monitoring add a layer of accountability. In places where management budgets for safer tools and pays attention to staff complaints, chemical accidents drop and workers stick around longer. Dilution isn’t the answer—smart design, good equipment, and a culture of care provide real protection against NMMO’s hazards.
Switching to alternative solvents has come up, but today’s cellulose chemistry keeps returning to NMMO for technical reasons. Until better options arrive, taking these hazards seriously protects not just individuals but public health and the waterways communities rely on.
Sitting behind a lab bench, you quickly learn not every chemical loves sitting out in the open air. That’s especially true for compounds like 4-Methylmorpholine 4-Oxide, Monohydrate, a solvent often used in cellulose processing and fiber spinning. Unpacking a drum of this material for the first time, I remember how humidity in the room shot up, even as the powder sat in its sealed bag. This compound doesn’t just react with water—it absorbs it like a sponge. If left on a shelf in a humid storeroom, you face a sludgy mess by the end of the week.
People with years of hands-on chemistry experience share one tip: treat this compound like dry pasta before cooking. Seal it tight, avoid contact with damp hands, and ignore any “good enough” shortcuts. It clumps fast when exposed to moisture, which turns weighing or transferring it into a challenging, messy task. If too much water sneaks into your supply, its effectiveness in dissolving cellulose drops sharply. A basic plastic lid does little to stop airborne moisture; you want thick, airtight containers that really close up and stay closed.
This compound won’t explode if you walk away for lunch, but that doesn’t mean you can toss it anywhere. Once, after storing it in a sunlit cabinet “just for a day,” I found sticky lumps forming well before the expiration date. N-Methylmorpholine N-oxide breaks down faster in heat. So avoid radiators, old window sills, or any spot with wide temperature swings. Find a stable, cool place—aim for under 30 degrees Celsius, but not so cold you get condensation problems. Keep it away from sunlight or open flames, as the safety data sheets from Sigma Aldrich and other reputable sources warn against fire risks. Fumes from burning this compound won’t do your lungs any favors; always keep fire extinguishers nearby when working with large amounts.
Desiccants make a big difference. Filling a small canister with silica gel and placing it in container corners has saved more samples than I can count. A good practice means not scooping the powder straight from the main drum, but dividing it into smaller, well-labeled bottles. This way, opening one container doesn’t risk the rest of the batch. For any operation where safety teams regularly check up, log each use and inspect containers for signs of softening or lumping. And always—always—store it in clearly marked, chemical-resistant containers meant for long-term chemical storage. Label each one with the date received, so you know which batch to use first.
OSHA and the European Chemicals Agency both offer strong advice about handling reagents like this. Stick with local chemical hygiene plans and wear proper protective gear. If a spill happens, don’t try to sweep up powder with bare hands. Use gloves, goggles, and, for big jobs, a respirator. Disposal needs to follow local laws—never just toss it in the trash or pour it down the sink. At every lab or plant I’ve worked in, the strongest safety culture grew from people respecting both written rules and the hard lessons of those who’ve handled these chemicals before.
Every ruined batch and every stuck pipette serve as reminders: proper storage protects not just the compound, but also the people and projects counting on it. Keeping 4-Methylmorpholine 4-Oxide, Monohydrate safe doesn’t need fancy technology or extreme costs. It takes common sense, airtight containers, and a respect for both chemical science and real-world experience.
4-Methylmorpholine 4-Oxide, Monohydrate often shows up on ingredient lists for making lyocell fibers. It works as a direct solvent for cellulose, which sets it apart from the acidic or harsh chemical methods used in older fiber-making techniques. You end up with strong, absorbent fibers like Tencel. These clothes last through repeated washing, manage moisture better, and hit the mark for folks who care about sustainability.
In textile factories, the push for safer and more eco-friendly production lines has given this chemical a clear role. Waste drops. Water use drops. Recovery systems cut down on the chemical lost in each batch, reducing environmental worries. Lyocell production claims almost closed-loop processing, and that matters with watchdog groups and environmental regulations tightening everywhere.
Besides textiles, this chemical lends a hand in labs and industrial settings as an oxidizing agent. That means it helps change one molecule into another, usually for specialty chemicals or drug development. Because it dissolves cellulose without breaking it down, scientists use it to study plant materials and develop biodegradable plastics or films. The ability to recycle and recover a large share of the solvent keeps costs in check, drives efficient pilot projects, and matches up with trends leaning towards greener chemistry.
I've seen mid-sized specialty chemical plants weigh the switch from more aggressive solvents to 4-Methylmorpholine 4-Oxide in their R&D lines. Cleaning up after experiments is easier and less risky for technicians. Lab managers report fewer headaches about local air quality rules thanks to its lower emissions compared to old-school volatile solvents.
This chemical opens doors for companies tackling sustainability goals without giving up performance. Regulations, especially in North America and Europe, push harder every year for cleaner manufacturing. Using 4-Methylmorpholine 4-Oxide cuts down the need for harsh chemicals like carbon disulfide, which not only pollutes but also endangers plant workers. It's easier to train new hires when materials carry fewer safety risks.
One challenge comes from sourcing and handling. The raw material for this solvent doesn't just pour out of nature ready to go; costs can jump around and disruptions hit quick. Engineers in production lines keep pushing for better solvent recovery. By recapturing and refining solvent from one batch to reuse in the next, plants save cash and cut waste by as much as 99%. Some R&D teams experiment with recycling streams, using closed-loop pumps and membrane filtration to strip out impurities for another run.
A few researchers have started to report promising results in using similar chemistry outside the textile sector, like in processing wood pulp to create tough, biodegradable packaging. They use small reaction vessels, cutting water and power demand, showing that even smaller players could get in the game.
Cleaner solvents make it easier for companies to attract responsible investors and meet rising customer demands for safety and sustainability. With 4-Methylmorpholine 4-Oxide, those moves don’t require building entirely new factories or suffering through lower product quality. Less pollution, safer workers, and a path toward recycling chemicals matter for any business hoping to thrive in industry’s next wave.
Working with chemicals always strikes up memories of lab coats, lingering odors, and the anxiety around making sure no small mistake turns into a headline incident. 4-Methylmorpholine 4-Oxide, Monohydrate, often used in specialty fibers and cellulose processing, stands out for its ability to dissolve cellulose better than many common solvents. Still, that power comes with a set of risks that can’t be shrugged off. I’ve seen mishandling of similar organic solvents go wrong in lab settings, where one missed precaution led to skin burns or shortness of breath.
Chemicals that pull apart cellulose so efficiently often show that same tenacity with organic matter like skin and lungs. Wearing the right gloves (nitrile, not latex) protects against burns and rashes. Splash goggles and full-length lab coats prevent splashes from reaching eyes and exposed skin. I’ve seen flippant about PPE pay the price with red, itchy hands by the end of a shift. Simple measures stop distractions, missed work days, and long-term damage.
Handling solvents in a busy space always risks fumes building up. Don’t rely on lucky breaks; always work under a chemical fume hood. I’ve seen workers stagger away coughing when ventilation fails for even a few minutes. Lingering fumes slow thinking and damage the lungs over time. Every bottle gets a tight, screw-on lid. No sense letting vapor escape or water get in. This chemical hydrates easily, and a little water turns it into gunge inside the bottle.
Even if it doesn’t flash into flames as easily as gasoline, 4-Methylmorpholine 4-Oxide can catch fire. Keep it away from open flames, hot surfaces, and static sparks. I once witnessed an ill-placed Bunsen burner turn a spill into a scare. Fire extinguishers should sit within arm’s reach, and every worker should know how to use them without reading instructions first. Never store it near oxidizing agents, since that can trigger dangerous reactions.
In labs and pilot plants, spills happen. Don’t freeze or trust rags and paper towels. Spill kits with absorbent pads, neutralizers, and personal protective equipment should sit nearby. Workplaces without a clear spill plan end up with panicked reactions and slips on slick floors. Quick response and containment keep minor issues from turning into emergencies.
Pouring chemicals down the drain never made sense. Collect waste in labeled, sealed containers and hand it over to a licensed disposal company. Waste streams should get tracked and reported so nothing disappears under the radar. Following local regulations helps avoid fines and keeps drinking water free of toxic leftovers. I’ve watched companies face real backlash after shortcuts led to leaks in local water supplies.
No one remembers every rule under pressure. Refresher training and clear, readable safety sheets posted in the lab room matter more than any policy document. New workers need hands-on practice, not just paperwork. Seasoned chemists benefit from peer reminders and visible checklists. These steps build good habits, saving lives and keeping everyone on the team accountable for safety.
Safe handling starts and ends with people who take every precaution seriously. In my experience, the best teams keep watch for corners left uncut. New regulations and advances in chemical engineering help, but personal responsibility remains the strongest defense against harm — both for workers and the environment they protect.
| Names | |
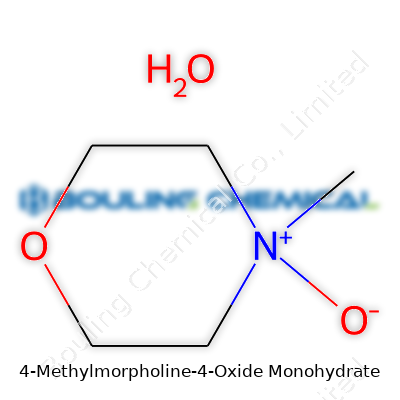
| Preferred IUPAC name | 4-methylmorpholine 4-oxide monohydrate |
| Other names |
N-Methylmorpholine N-oxide monohydrate NMMO monohydrate N-Methylmorpholine oxide monohydrate 4-Methylmorpholine N-oxide monohydrate |
| Pronunciation | /ˈfɔːr ˈmɛθɪlˌmɔːfəˌliːn ˈfɔːr ˈɑːksaɪd ˌmɒnəˌhaɪdreɪt/ |
| Identifiers | |
| CAS Number | 7529-22-8 |
| 3D model (JSmol) | `6fcmMolOCOC1CN(CCO1)C` |
| Beilstein Reference | 127873 |
| ChEBI | CHEBI:131056 |
| ChEMBL | CHEMBL1233095 |
| ChemSpider | 156346 |
| DrugBank | DB14642 |
| ECHA InfoCard | ECHA InfoCard: 100.041.864 |
| EC Number | 216-635-6 |
| Gmelin Reference | 105131 |
| KEGG | C14343 |
| MeSH | D017239 |
| PubChem CID | 86289073 |
| RTECS number | QD1050000 |
| UNII | 5KRJ6JIE2V |
| UN number | “2811” |
| Properties | |
| Chemical formula | C5H11NO2·H2O |
| Molar mass | 121.16 g/mol |
| Appearance | White crystalline powder |
| Odor | Amine-like |
| Density | 1.032 g/mL at 20 °C |
| Solubility in water | soluble |
| log P | -1.6 |
| Vapor pressure | <1 mmHg (20 °C) |
| Acidity (pKa) | 7.8 |
| Basicity (pKb) | 7.92 |
| Magnetic susceptibility (χ) | -6.3e-6 cm^3/mol |
| Refractive index (nD) | 1.485 |
| Viscosity | 4.5 mPa.s (20 °C) |
| Dipole moment | 5.45 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 213.7 J/mol·K |
| Std enthalpy of formation (ΔfH⦵298) | -531.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2102 kJ/mol |
| Pharmacology | |
| ATC code | V20AB08 |
| Hazards | |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H319 |
| Precautionary statements | P261, P264, P271, P273, P280, P302+P352, P305+P351+P338, P304+P340, P312, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 2-1-1 |
| Flash point | 93 °C |
| Autoignition temperature | 215 °C (419 °F) |
| Lethal dose or concentration | LD50 Oral Rat 4570 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 1060 mg/kg |
| NIOSH | RN 7529-22-8 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 0-50 mL/L |
| Related compounds | |
| Related compounds |
N-Methylmorpholine N-Methylmorpholine N-oxide 4-Methylmorpholine Morpholine Morpholine N-oxide |