Digging through old chemistry books, anyone can see how humble beginnings often mark the road for many now-common chemicals. 4-Methylimidazole, or 4-MEI, has a backstory rooted in late 19th-century research into heterocyclic compounds, when scientists craved answers about why heat changes the stuff in plants and animals. Decades ago, folks weren’t worried about what popped up in caramel coloring—they just wanted better dyes and corrosion inhibitors. As industry got busier, people expanded the catalog of imidazoles, testing traces everywhere from coffee to rubber. These days, anyone paying attention can see how academic curiosity led this molecule out of the lab and straight into both big manufacturing lines and controversy.
4-Methylimidazole shows up in processes where heat and ammonia mix with sugars. As a non-natural byproduct of browning reactions—think caramel coloring in soda, roasted coffee, and even cigarette smoke—the stuff proves how chemistry never stops at theory. Commercial production often leans on its value in syntheses, crop protection chemicals, pharmaceuticals, and pigments. In a world where branding and blame land on labels, the presence of 4-MEI stirs spirited arguments about how food processing plays out behind closed doors.
This compound doesn’t stand out. 4-MEI is an off-white to yellowish solid at room temperature. It dissolves in water, giving off a faintly ammoniacal smell—nothing that surprises a lab worker used to imidazoles. Its melting point sits near 45°C, with a boiling point just over 260°C, keeping it manageable in most facilities. The thing that matters most for product safety is its affinity to bind with acids and its basic nitrogen atoms, which make it a fine intermediate for tweaking other chemicals. Shelf life turns out decent—store it dry and cool, and there’s little drama.
Any company shipping loads of 4-MEI must meet critical guidelines. Package labels give CAS number 822-36-6, highlight imidazole content, moisture, and heavy metal levels, and carry hazard symbols. Regulations from countries like the US and EU push firms to keep residuals minimal, especially in foods and pharmaceuticals. That’s not a formality—watchdogs often test for 4-MEI down to the microgram, since consumer health and legal battles hang in the balance. This focus shows how technical standards reflect larger attitudes about trust and transparency.
Labs usually rely on the Radziszewski reaction, a workhorse process for making 4-MEI. This approach bolts together methylglyoxal, ammonia, and formaldehyde in water—heat it up, and you’re left with more product than byproducts. Some manufacturers tweak solvents or temperatures to boost purity or cut cost, but the recipe rarely strays far from these basics. That’s because consistent preparation methods mean fewer surprises for safety testing and easier planning for scale-up. After all, no engineer wants to reinvent the wheel for a commodity chemical.
Chemists treat 4-MEI as a platform for making all sorts of new molecules. Basicity at the ring nitrogen means it can grab at electrophiles—handy for making pharmaceuticals or boosting performance in epoxy resins. Add a little halogen or strong acid, and the methyl group provides a handle for more substitutions. People in crop science use these traits to link the ring to bigger pesticide molecules. So the story here is flexibility—4-MEI moves easily from starting material to specialty compound because its core structure lends itself to modification.
Trade and academic circles swap a handful of names for 4-MEI. You’ll see it as 4-methylimidazole, 4MI, or sometimes 1H-imidazole, 4-methyl-. A quick look through regulatory lists brings up synonyms like 4-Methyl-1H-imidazole and 822-36-6 (its CAS registry). Don’t get thrown off if you spot brands jazzing up the label for a specialty blend—at the molecular level, it’s all the same stuff.
Safety officers don’t take shortcuts with 4-MEI. Occupational guidelines say to avoid direct contact—this isn’t sugar or flour. Gloves, goggles, and half-face respirators form the daily uniform when measuring, mixing, or cleaning up spills. Inhalation or skin exposure causes irritation, so engineers run ventilation fans and keep first aid ready. Storage calls for sealed drums away from oxidizers and acids. In places with stricter laws, companies run exposure monitoring on workers, and regular training stands between complacency and a workplace incident. All these layers reflect hard lessons learned whenever regulations grew teeth after an accident.
Industry treats 4-MEI like a versatile cog in big machinery. It supports the pharmaceutical sector as a building block for treatments that battle diseases or regulate hormones. Dye makers find it essential in stabilizing color and boosting lightfastness. Agribusiness relies on it for crop protection agents—powerful molecules that keep fields productive. Electronics companies stick with this compound for specialty resins, which perform under heat or stress. Even food processors stare at test results for 4-MEI since caramel coloring, a common additive, brings it along as an uninvited guest. Each of these uses throws the compound into the intersection of safety, science, and society’s shifting attitudes.
R&D teams keep tinkering with process improvements, cleaner syntheses, and detection methods for 4-MEI. Instrumentation now tracks parts per billion in factory emissions and finished foods—no more flying blind about what’s really inside a sample. Universities ask pointed questions about reaction mechanisms and biological impacts, aiming to shrink risk and uncover new uses. Creative minds at specialty chemical companies explore greener production with renewable feedstocks, hoping to curb environmental concerns or sidestep stricter legislation. This push for smarter science puts pressure on both big firms and small startups to innovate for both profit and public good.
Toxicologists put 4-MEI under the microscope after spotting it in caramel coloring. Animal studies link high doses to possible cancer risk, prompting California to slap warning labels on foods and drinks with more than a smidge. Regulatory agencies like the FDA and EFSA reviewed reams of data. They accept that exposure from cola or coffee in ordinary diets lands way below concerning thresholds for most folks. Still, the uncertainty fuels debate between industry and consumer groups. Food makers lowered 4-MEI content by adjusting processing methods; testing keeps everyone honest. People’s gut reactions to risk and regulation reveal how trusted science depends on open communication and willingness to adapt.
4-MEI faces forks in the road as science, law, and markets evolve. As more automation and process control settle into manufacturing plants, exposure risks drop, and detection moves from specialty labs to everyday operations. Regulatory walls get higher for food and pharma players, forcing process tweaks or outright substitutions in caramel coloring and coatings. Some research hints at potential in medicinal chemistry, as a stepping stone toward cancer drugs or antiviral agents—opportunities ride on how well toxicity gets managed or sidestepped. Green chemistry provides a north star for cleaner routes, perhaps using biobased precursors. What comes next depends less on laboratory breakthroughs and more on coordinated efforts between scientists, regulators, producers, and an increasingly health-aware public.
Grab a bottle of cola, take a look at brown sauces, or peer at some baked goods, and the chemistry hiding in the background becomes pretty clear. 4-Methylimidazole, usually shortened to 4-MeI, has found its way into more than labs and factories—it hides out in lots of foods and drinks, thanks to the way many products are colored or processed.
Inside the ingredient list, you might spot “caramel color,” which sounds innocent enough. That’s where 4-MeI pops up most often. Caramel coloring, especially the kind made using ammonia and sulfite, creates this chemical as an unintended byproduct. Soft drinks get their deep hue thanks to this process.
Plenty of chemical makers create 4-MeI in larger quantities for other uses, too. Industrial processes use it for pharmaceuticals and some agriculture chemicals. It acts like a building block in those cases, helping scientists string together more complicated molecules. Some companies lean on 4-MeI as part of photographic chemicals, dyes, and rust inhibitors.
The link back to food still grabs the most attention, mainly because people end up consuming it without ever asking for it. You can cook a steak or bake bread at high heat and produce it, too. It’s not only industry—regular kitchen habits can make tiny bits of 4-MeI.
Sometimes a big word can spark debate. 4-Methylimidazole punched through the headlines after studies in the early 2000s flagged it as a possible risk. Animal studies suggested a link to cancer; in the U.S., California added 4-MeI to its Proposition 65 list, making companies warn customers if their products contain high levels. That set off a wave of questions—do soft drinks actually pose a real risk?
Later studies in people haven’t drawn a clear line between typical dietary exposure and health problems. The U.S. Food and Drug Administration hasn’t cracked down on the caramel coloring itself, but the story highlights a real challenge. Even tiny traces of a chemical in a food or drink can spark a lot of concern, and science doesn’t always move fast enough for those answers to land before worry sets in.
With enough consumer pressure, big soda companies began lowering the levels of 4-MeI in their caramel coloring. They tweaked recipes or worked with suppliers to change the process, especially for products sold in California. Testing improved, and more labs started checking for 4-MeI in food and drink products.
Regulators face tough choices. Banning caramel coloring across the board doesn’t make sense for every product. Setting limits, making sure companies run regular tests, and pushing for cleaner production methods have made more of an impact than one-size-fits-all rules. People want to know their food won’t harm them, but they also want choice.
As a shopper who likes to read labels—sometimes obsessively—seeing “caramel color” sparks a little moment of doubt. Still, better transparency, stronger regulations, and the push from public health groups have nudged things in the right direction. While science keeps chasing down better answers, it’s not a bad idea to know what’s in your snack, your sauce, or your soda. Asking those questions has already shifted the market.
I’ve always wondered how snacks get their colors and flavors. Flip over a bottle of cola or a bag of caramel corn, and you’ll spot a list of ingredients that read like something from a science lab. 4-Methylimidazole pops up in this world. It forms when manufacturers cook certain sugars and ammonia at high temps, leading to that signature golden-brown color in sodas and sauces. If you ever tried making your own caramel on the stove, you created trace amounts, too, without even noticing.
News stories love scary words. They tell us 4-Methylimidazole has links to cancer—in lab animals at least. California slapped a warning label on products with high levels, making shoppers stare and worry. I’m not one to panic over every headline. But as a parent and a coffee drinker, I pay attention when authorities bring up toxic risks that sneak into everyday food.
The truth hides deeper than a flashy headline. Rodents that developed cancer in studies got doses far higher than anything I’d find in my breakfast. Looking at actual food, the US FDA checked levels in soft drinks and found they usually sit far below amounts health groups think raise any real risk. If you drink cola every now and then or use some browning sauce in stir-fry, your risk doesn’t jump off the charts.
At the same time, California’s “Prop 65” law takes a hard line, forcing companies to stick a warning on drinks with more than 29 micrograms of 4-MEI per day. This made soda manufacturers rethink recipes for that market. Funny enough, tests show that sodas sold in California usually have lower 4-MEI levels than those in other states—proof that laws can nudge companies to tweak things for safety, even if the actual danger isn’t clear-cut.
It’s all about context. Swallowing a shot glass of pure 4-MEI sounds reckless, but that’s not the way folks interact with additives. In my own life, I’ve seen people get more harm from sugar and caffeine than from the trace chemicals spun up in flavorings. Balance matters way more than trying to dodge every chemical with a scary name.
Europe and the US both monitor new research. If they found solid proof that caramel coloring packed a punch at real food levels, they wouldn’t keep quiet. Scientists know people eat differently, so they test foods in lots of ways and cover their bases with safety margins that run wide.
Still, bigger companies know shoppers demand cleaner labels. Some food brands today boast of “no caramel coloring” just to reassure, not because they think it stops cancer. I often choose foods with fewer artificial bits, not because I fear specific chemicals, but it helps me recognize what I’m eating. More transparent labeling—without overblown fear—gives everyone a real shot at making choices they trust.
Anyone worried about food chemicals gets stuck between two extremes: either panic at every new “carcinogen” or dismiss all chemical worries as nonsense. Facts land somewhere in between. I don’t spend my life scared of cola, but I’m glad someone’s watching out, testing, and raising the bar for what goes in our food. That’s not some grand fix, but it makes me feel a bit safer about what hits my plate.
4-Methylimidazole, or 4-MEI, often shows up in industrial labs and sometimes even in food production, thanks to its role in chemical manufacturing. This compound isn’t just another bottle on the shelf—it carries health concerns, with links to potential toxicity and even questions around cancer risk in certain animal studies. Stories from chemistry labs stick out in my mind, where leaky seals and poor planning turned minor spills into reasons for evacuation. One thing’s clear: safe storage isn’t just a checkbox. It’s the backbone of good lab practice.
4-MEI likes stability—and so do those who store it. Let this material warm up or get damp, and you open the door to decomposition or even chemical reactions you never meant to start. Direct sunlight can push the temperature up enough to change its physical state. Humidity brings in water, which can alter the way certain chemicals behave.
A dedicated, well-ventilated cabinet earns its keep in any workspace handling organic compounds. I’ve seen the difference a solid, ventilated metal cabinet makes, especially compared to old, wooden cupboards that trap fumes and set up long-term problems. A room kept between 15°C and 25°C works for most situations—nothing fancy, just out of the sunlight and away from any steam or heat sources.
Storage gets especially tricky in busy labs where shelves fill up fast. Mixing 4-MEI with acids or oxidizers spells trouble, sometimes even fire. Years ago, I heard about a chemistry storeroom where acids sat just above organics, and a single broken flask sent bottles crashing together. After that, the department rewritten its handbook: organics like 4-MEI only line up with other compatible, stable compounds—not with strong acids, peroxides, or anything that might trigger a reaction. A simple segregated storage plan goes a long way here, using clear labeling and physical barriers, never leaving safety up to chance.
Glass with tight-sealing lids usually works best for solid 4-MEI. Over time, plastic can break down or let vapor seep out, so heavy-duty borosilicate glass offers extra safety. I learned never to trust a cracked jar or an old, soft rubber stopper—one failed seal can send up that bitter, musty smell, meaning contamination already started. Always double-check for corrosion or residue before reusing any jar.
Every bottle should carry more than just a name. Labels tell users about concentration, hazard level, and the date opened. After once discovering an old, dusty bottle with a faded tag, I always make sure dates stay visible and legible. This habit means no mystery chemicals and keeps everyone on the same page during audits or emergency cleanups.
Plans matter most in the moments you hope never come. An up-to-date spill kit—absorbents, gloves, goggles—should stay nearby, but not buried under clutter. Fast access stops minor mishaps from turning into all-hands disasters.
Open conversations about storage routines don’t just protect users—they keep the whole operation running smoothly. I’ve seen—more than once—how regular checks and real-world safety drills catch problems before they grow. Making safe storage a team effort turns best practices into everyday habits, without extra stress or wasted time.
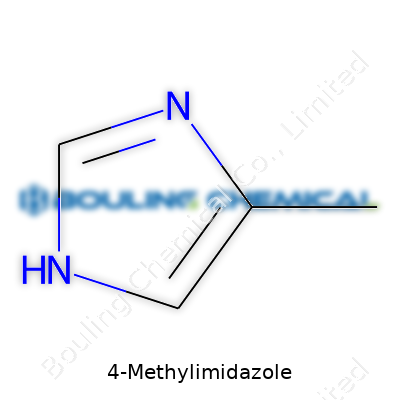
4-Methylimidazole doesn’t get much fanfare outside chemistry circles, but it pops up everywhere from caramel coloring in soda to certain medications. Without a complicated backstory, this compound is just an organic chemical with an interesting punch. Its structure isn’t exotic: a five-membered ring, holding on to four carbon atoms and two nitrogens. Add a simple methyl group (that’s just one carbon and a few hydrogens) and suddenly you’ve got 4-Methylimidazole, or 4-MEI for short.
Pull a vial of this stuff from a lab shelf and you’re met with needle-like crystals or maybe a dusting of a tan solid. It doesn’t drift up your nose, because the smell just isn’t strong. Clocking in with a melting point near 44°C–46°C, it doesn’t stick around solid for long if you hold it in a warm hand. If you're trying to dissolve 4-MEI, water does the trick pretty well—this sets it apart from more stubborn organic molecules that barely mix with water at all. Plus, mix it with alcohol or acetone and it’ll disappear right in.
What really sticks with me is how this compound refuses to flare up at the first sign of heat. Its boiling point sits at around 267°C, so it sticks together well past the boiling point of water. You won’t catch it evaporating on a summer day in the lab or kitchen.
You find 4-MEI sitting comfortably as a weak base. Its nitrogen atoms can pick up a proton, but most acids still outdo it in that department. That slight basicity changes up how it behaves in water—it doesn't just float around, it interacts, sometimes helping catalyze reactions that would otherwise dawdle along.
In my time mixing chemicals, I’ve seen how 4-MEI charms other molecules with its nitrogens—able to form hydrogen bonds, drawing things together like a good barista with steamed milk and coffee. That hydrogen bonding brings practical effects, giving it a hand in forming stable complexes with metals or acting as a stepping stone in chemical syntheses. Leave it on a shelf and it won’t break down in a hurry; it stays tough under ordinary temperatures, nothing dramatic unless strong acids or oxidizers get involved.
Chemically, the methyl group hanging off the ring doesn’t just change the name, it tweaks the reactivity. It makes 4-MEI less likely to act like plain imidazole. You get subtle differences in how it takes part in reactions involving other organic molecules. That’s why industries keep coming back to this molecule’s toolkit.
4-MEI has gotten plenty of attention in food science and public health circles. Food processing can bring it in as a byproduct, especially when producers caramelize sugars. Some research on animals has waved red flags about its potential as a carcinogen. These studies spark debates about regulations and acceptable exposure. California lists it under Prop 65, so food companies keep eyes on its levels in sodas and sauces.
We can’t just brush off what we eat or drink—especially when it drags along chemicals like this. Better testing in food, deeper studies into long-term exposure, tighter limits and honest labeling bring some peace of mind. For chemists in the lab, lab coats and gloves keep things safe during handling, but the bigger question sits with how the stuff lands in our daily lives. This isn’t just chemistry—it’s a question about safer choices and smarter oversight.
4-Methylimidazole pops up in more places than you might think—mostly in manufacturing, sometimes as a byproduct in food, especially anything with caramel coloring. Controversy around it started after links between the chemical and certain health risks, like cancer in lab animals, sparked tighter regulations. California’s Proposition 65 flagged it as a possible health concern. So, when this stuff shows up in the workplace, a casual attitude just won’t cut it.
Let’s say a bottle slips from your hand, and suddenly 4-MEI splashes on the lab floor. No time to panic—time to act. First move: clear the area, make sure nobody strolls in without protection, and reach for the proper gear. At my old job on a chemical plant floor, we wore nitrile gloves and worked under a fume hood for a reason. 4-MEI loves to drift around in the air, and inhaling dust isn’t something I’d risk.
A responsible response uses more than mop and hope. Scoop up any solid, seal it tight in a container, and label it. Sweep, but skip the broom—use a HEPA vacuum to pick up powder without sending it airborne. If it lands on a bench or a solid surface, wipe it up with a damp cloth, then get rid of the cloth like hazardous waste. Pour anything liquid into a secure container for disposal. No rinsing down the sink; local rules usually ban this for good reason.
Personal exposure changes everything. I once watched a colleague brush chemical dust off bare skin and pay the price with a nasty rash. With 4-MEI, wash skin with soap and lots of water—don’t just dab it off. If any lands in your eye, rinse it under running water for a good fifteen minutes, even if it stings like mad. Throw your clothes in a sealed bag and hit the shower. If breathing gets tough or you feel sick, head straight to the doctor. Don’t just tough it out.
Safe storage speaks louder than any sign on the wall. I’ve seen corners cut—chemicals stored without labels, safety data sheets missing from files. That’s asking for trouble. Keeping chemicals in the right containers, labeling everything, and making sheets easy to find prevents mix-ups and dangerous accidents. Good ventilation in workrooms and spill kits on standby shrink the risk before anything goes wrong.
A lot of spills happen because someone new hasn’t seen a spill drill or never paid attention in safety training. Having clear rules, not just posters, helps everyone stay alert. In-house drills and honest talks about what went wrong after an accident teach more than a page of instructions. Simple reminders—check your gloves, double-check your labels, keep the gear close—keep small mistakes from growing into big ones.
I’ve seen both careful teams and careless shortcuts. Good habits, supported by the right equipment and real practice, keep workplaces and workers safe. 4-Methylimidazole isn’t about lab fears or headlines. It’s about people coming home from their jobs as healthy as they arrived.

| Names | |
| Preferred IUPAC name | 4-Methyl-1H-imidazole |
| Pronunciation | /ˈmɛθəlɪˌmɪdəˌzɒl/ |
| Identifiers | |
| CAS Number | 822-36-6 |
| Beilstein Reference | 1209224 |
| ChEBI | CHEBI:28241 |
| ChEMBL | CHEMBL12393 |
| ChemSpider | 5799 |
| DrugBank | DB06802 |
| ECHA InfoCard | 03e74f21-16c2-4459-8eaa-7a5c82b2ceaf |
| EC Number | 211-765-7 |
| Gmelin Reference | 83488 |
| KEGG | C02289 |
| MeSH | D008974 |
| PubChem CID | 6969 |
| RTECS number | MN0175000 |
| UNII | I1M7Z3840P |
| UN number | 2810 |
| Properties | |
| Chemical formula | C4H6N2 |
| Molar mass | 82.11 g/mol |
| Appearance | White to light yellow crystal or powder |
| Odor | Ammonia-like |
| Density | 1.03 g/cm³ |
| Solubility in water | Soluble |
| log P | -0.41 |
| Vapor pressure | 1 mmHg (at 20 °C) |
| Acidity (pKa) | 7.05 |
| Basicity (pKb) | 7.06 |
| Magnetic susceptibility (χ) | -70.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.488 |
| Viscosity | 2.12 mPa·s (25 °C) |
| Dipole moment | 1.37 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 227.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -23.19 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3829 kJ/mol |
| Pharmacology | |
| ATC code | V03AB37 |
| Hazards | |
| Main hazards | Harmful if swallowed; causes serious eye irritation; may cause cancer. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P261, P264, P280, P301+P312, P304+P340, P312, P330, P405, P501 |
| Flash point | 56°C |
| Autoignition temperature | 444 °C |
| Explosive limits | 2.7% - 15.2% |
| Lethal dose or concentration | LD50 oral rat 1180 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 4-Methylimidazole: 570 mg/kg (oral, rat) |
| NIOSH | NIOSH: PH9890000 |
| PEL (Permissible) | 5 ppm |
| REL (Recommended) | 500 mg/m³ |
| IDLH (Immediate danger) | 300 ppm |
| Related compounds | |
| Related compounds |
Imidazole 2-Methylimidazole Histidine |