Back in the early days of industrial chemistry, scientists kept their eyes peeled for versatile molecules that could help push boundaries. 4-Methylbenzothiazol-2-ylamine, a derivative of benzothiazole, first surfaced during the rapid expansion of sulfur- and nitrogen-containing aromatic compounds in the last century. Chemists, often working by trial and error, sought alternatives to previously difficult or expensive compounds. The roots of this molecule can be traced to the development of dyes and pharmaceuticals, where modifications to the benzothiazole skeleton led to improved colorfastness or medicinal effects. The spread of structural knowledge through analytical tools like NMR and mass spectrometry in the 1960s drove sharper insight, so what used to be just a lab curiosity quickly earned a solid spot on the bench—especially as a building block for more complex chemicals.
4-Methylbenzothiazol-2-ylamine demonstrates both versatility and utility in many labs. Chemists working on high-performance dyes, biologically active ingredients, or specialty sensors find it reliable. Its chemical backbone, benzothiazole, has proven adaptable, and the methyl and amine groups crank up its reactivity and compatibility with diverse chemical reactions. Sourcing it no longer tests patience, as manufacturers routinely offer solid, well-documented lots—sometimes in bulk for industrial scale-up, sometimes in smaller, lab-ready jars for research.
The compound arrives as a yellow to light brown crystalline powder. Solubility veers toward organic solvents like ethanol, acetone, and chloroform, but water usually proves tough. Melting points congregate between 147°C and 152°C, showing consistency across reputable suppliers. The aromatic system stabilizes the core, supporting gentle handling under standard laboratory conditions. Chemical stability stands out—methyl and amine groups don’t cause trouble at room temperature, but the ring structure may complain under strong oxidation or reduction. The amine group adds nucleophilic personality, making the molecule responsive during coupling or substitution reactions.
Standard packaging for 4-Methylbenzothiazol-2-ylamine often lists purity between 97% and 99%, based on HPLC or GC analysis. Labels show batch number, date of manufacture, and safety pictograms. SDS documentation covers potential risks, so users don’t have to scramble during audits. Producers make sure to indicate country of origin, shelf life (typically 24 months under cool, dry storage), and storage recommendations—often stating to keep it away from strong acids, oxidants, or direct sunlight to prevent degradation.
Long-standing synthesis routes take advantage of ease of substitution on the benzothiazole ring. A preferred method uses 4-methylaniline, which reacts with carbon disulfide and bromine to cyclize into 4-methylbenzothiazole. Careful amination follows, typically with ammonium carbonate under controlled temperature. Process tweaks help maximize yield and minimize unwanted byproducts. Many labs have tried green chemistry routes using catalytic oxidizers or microwave irradiation, shaving down cycle times and resource waste. Large-scale manufacturing optimizes solvent recovery and reaction energy, both to save money and meet growing regulatory demands for sustainable production.
4-Methylbenzothiazol-2-ylamine thrives in both small-scale synthesis and industrial upscaling. Its amine group makes a strong partner for acylation, sulfonation, and alkylation, creating links to a variety of larger molecules. Suzuki and Heck couplings slot right in to modify the aromatic ring or to build complex scaffolds for pharmaceuticals. Derivatives touch several industries—with dye manufacturers modifying it to shift color hue, and pharma labs tweaking it to pursue new antimicrobial or anti-inflammatory agents.
Purchasing and regulatory paperwork often mention several names for the same compound, so cross-referencing matters. The most common synonyms include 4-methyl-2-aminobenzothiazole, 2-aminobenzothiazole-4-methyl, and CAS number 2508-56-7. These alternate names help researchers sidestep confusion, especially during international collaborations or dealing with multinational suppliers. Some catalogues also list custom product codes, always tying back to the original chemical structure.
Lab users learn to handle 4-Methylbenzothiazol-2-ylamine with conventional PPE—nitrile gloves, lab coats, and splash goggles. Safety Data Sheets point out the irritant nature of dust, which can bother eyes and mucous membranes on contact or inhalation. Standard storage calls for tightly sealed containers under nitrogen or dry air, steering clear of moisture to prevent hydrolysis or unwanted oxidation. Fire risks stay low, but dry powder extinguishers and familiar emergency protocols come in handy. Environmental regulations dictate clear disposal routes for unused material and waste—most often through incineration at authorized facilities.
Dyes stand out as perhaps the largest consumer of 4-Methylbenzothiazol-2-ylamine, lending brightness and lasting color to textiles and specialty inks. Specialty chemical synthesis taps into it, spinning out intermediates for agrochemicals, medicines, and even advanced electronic materials. Pharma researchers hunt for analogues as enzyme inhibitors or molecular imaging agents, where electron-rich aromatic systems improve binding in complex biological systems. Polymer chemists sometimes feed it into specialty resins to enhance reactivity, making coatings or adhesives with custom features.
Recent years brought a surge of research focused on tweaking this scaffold. Chemists search for new derivatives that block microbial growth or slow tumor cell division. Analytical chemistry circles exploit fluorescence properties, using sensitive probes built from benzothiazole cores—including 4-Methylbenzothiazol-2-ylamine modifications—for DNA or protein detection. Scientists also spend time modeling structure-activity relationships, running computational screens to shave months off development cycles. Collaboration between chemical suppliers and universities helps drive proof-of-concept work into real-world applications, sometimes spinning off joint patents or open-source datasets.
Most standard toxicity screens rank 4-Methylbenzothiazol-2-ylamine relatively low on acute hazards, but eyes and skin feel irritation fast after direct exposure. Animal studies suggest limited systemic toxicity, with oral LD50s typically higher than those considered immediately dangerous. Long-term studies, especially on chronic exposure and metabolic breakdown products, remain patchy—which leaves occupational safety coordinators on high alert. Tight air controls, personal monitoring, and regular medical exams become the norm in facilities handling large volumes. Regulatory agencies keep pushing for expanded biological and environmental fate data, particularly for compounds ending up in water supplies or bioaccumulating in food chains.
Interest in this molecule looks set to grow as green chemistry and specialty material demands keep rising. Energy-efficient synthetic methods show promise—chemical engineers target continuous flow reactors, reducing waste and using less energy. Applications outside traditional dye and pharma markets emerge, from organic LEDs to smart sensors and markers. Data from ongoing toxicity trials and environmental monitoring will shape use protocols, possibly opening the door to fully biodegradable derivatives. As industry focus shifts toward sustainability and safe chemical handling, research groups face the challenge of balancing utility, safety, and environmental responsibility, all without rolling back the progress tied to this once obscure aromatic amine.
4-Methylbenzothiazol-2-ylamine tends to show up in conversations about dyes, pigments, and even rubber production. Most people never hear that name outside a laboratory or manufacturing setting. Still, the chemical world we rarely see keeps much of modern life humming along, and this is one of those behind-the-scenes helpers.
People riding on car tires have 4-Methylbenzothiazol-2-ylamine in the background. Factories use derivatives of this compound as part of a class of accelerators, speeding up the vulcanization process—what transforms sticky rubber sap into bouncy, durable tire tread. Long ago, making strong rubber meant heavy air pollution and toxic waste, but new chemical routes have cut down on those problems. This compound fits into the changing landscape of safer, cleaner rubber production, at least compared with methods from the past century.
Anybody who has walked through a textile mill or taken a whiff as clothes are colored might catch traces of benzothiazole compounds at work. They often sit at the core of bright dyes—especially ones used for silk, wool, or specialty plastics. Dye-makers tweak molecules like 4-Methylbenzothiazol-2-ylamine to get greens, blues, and violets that last through washing and sun exposure. My first real memory of playing with tie-dye as a kid brings back the wild chemical stink from the kitchen sink—and reading up later, I realized those vibrant colors owed their “pop” to a handful of finely tuned chemicals.
Not everything is rosy, though. Evidence links benzothiazole compounds to water pollution in rivers near industrial hubs. Some byproducts persist and build up in fish, and researchers have found them in stormwater runoff around big cities. Long-term studies have started to ask tough questions about chronic exposure. Lab tests with large doses showed effects on aquatic life, so environmental scientists advocate for better treatment of industrial waste and stronger monitoring of effluents.
On the human side, professionals in dye and chemical plants need strong training and the right protective gear. Mishandling any chemical raises risks, but this family has shown enough toxicity in animals to cause restrictions in some settings. For everyday consumers, normal use of products—clothes, tires, electronics—does not create notable danger, as the chemical gets tightly bound inside finished objects.
The story is not just a warning. Tracking and limiting industrial leaks has succeeded in many countries. Cleaner technologies—like closed reactor systems and advanced wastewater treatment—can slash the traces of these compounds that escape into streams. At the consumer level, more companies have begun to ask suppliers about the origin and kinds of accelerators and dyes they use, pushing for transparency all down the chain. It reminds me of recent conversations with sustainability-focused colleagues. They point out that real progress often comes not from new laws but from regular people asking for real answers about what’s in their products.
As science keeps moving, safer alternatives will show up—and so will stronger oversight. For now, 4-Methylbenzothiazol-2-ylamine lives quietly in the chemistry of products we use, part of a story that stretches from industry floor to environmental watchdogs and, ultimately, to everyone who depends on sturdy tires or vibrant color.
4-Methylbenzothiazol-2-Ylamine carries a molecular formula of C8H8N2S. Years spent in chemistry labs taught me the practical value of knowing a compound’s exact formula. It’s more than textbook trivia. Every researcher, formulator, or regulatory expert depends on precise information at the molecular level. One carbon or hydrogen out of place means a new substance with different hazards, features, even behavior in the environment or the human body.
Start naming unfamiliar molecules in front of a room full of chemists, and questions fly: “How many nitrogens? Is there a sulfur elsewhere?” Without a solid molecular formula, clear discussions stagger to a halt. When I was running HPLC experiments, a missing atom in the record could throw off identification or lead to errors in analyzing results. Chemical formulas grant the confidence to replicate results, store data, and compare findings across different teams worldwide.
Knowing that 4-Methylbenzothiazol-2-Ylamine is C8H8N2S reaches far beyond academia. Regulatory authorities, like OSHA or the EPA, demand precise details before companies can manufacture, import, or use chemicals for new purposes. Incorrect or incomplete information often means downtime, returned shipments, audits, or worse—an unsafe workplace. My experience assisting with safety data sheets showed how a missed nitrogen led to legal drama between a supplier and a customer. Sometimes, the difference in paperwork caused by a single atom’s absence brought millions in fines or product recalls.
Industry needs formulas to develop safe handling procedures, fire protection plans, and emergency response protocols. C8H8N2S tells them about flammability, possible decomposition products, necessary personal protective equipment, and safe disposal. Engineers, not just chemists, depend on this data for storage, transfer, and waste treatment systems. For anybody running a plant or a lab, forgetting the basics costs real money and sometimes, puts people in danger.
Quality research rests on rock-solid records. Peer-reviewed publications, patents, and grant applications check for correct identification before review. When scrambling to write or read a paper, nothing stings more than finding a structure missing a methyl group or sporting an extra sulfur. Experiments that worked for one group fail in the next lab if the molecular identification is off. Blindly scaling up a synthesis based on hearsay carries real risks and wasted effort.
More than once, research projects stalled over inconsistencies in basic details. Reproducibility comes less from fancy equipment and more from rigor with simple fundamentals—the right formula, structure, and understanding of what stands in the flask. C8H8N2S doesn’t just describe a molecule, it marks the launchpad for every synthesis, analysis, and application downstream.
Accuracy in chemical naming and structure comes down to discipline in recordkeeping, double-checking with trusted databases, and following established naming conventions. Digital tools and chemical informatics help, but nothing replaces an engaged human eye. Students, managers, and researchers benefit from regular training. I’ve seen teams avoid costly mistakes just by taking five minutes to review chemical formulas before ordering reagents or beginning new research. Small steps like cross-verifying details, using molecular visualization software, and sharing clear records create a culture of safety and transparency.
So, with its formula of C8H8N2S, 4-Methylbenzothiazol-2-Ylamine stands as a reminder that big progress depends on paying attention to even the tiniest fragments. For all kinds of science and industry, the details really matter.
Chemicals with hard-to-pronounce names often pop up out of nowhere, sparking all sorts of questions about safety. 4-Methylbenzothiazol-2-ylamine stands out among these, not because it appears in grocery products, but because it drifts through the world of industrial chemistry. Recognizing the hazards linked to this substance matters for anyone working near it, whether on a factory floor or in a research lab.
Testing shows some chemicals aren't friends to human cells. Peer-reviewed studies point out that benzothiazole compounds can lead to harmful effects. With 4-methylbenzothiazol-2-ylamine, the benzothiazole ring plus the amine group create an odd couple, raising toxicity flags. Research on closely related chemicals shows links to skin and eye irritation, organ damage in rodents, and issues with long-term exposure. The European Chemicals Agency flags substances like this for regular observation, mainly because repeated handling over months or years leads to accumulation in the body.
Anyone who's spent time in a lab learns respect for chemicals fast. Touching unknown powders with bare hands comes with risk. The moment a strong chemical smell hits, scientists switch out latex gloves and open every vent. Sometimes, workers experience rashes, sneezing, or headaches just after exposure to aromatic amines. Add up these moments, and you don't need a degree to figure out that repeated, even short, exposure could mess up a person's health.
OSHA and similar groups offer clear standards. Wearing gloves, using goggles, and sticking to chemical fume hoods keep these risks in check. Small steps like good labeling and regular training sessions matter. If a chemical causes mouth or eye stinging once, it often does it again. Too many ignore the signs until it's too late, thinking small spills or dust clouds present no problem.
Environmental fallout rarely gets the spotlight it deserves. Benzothiazole derivatives tend to stick around in water and soil, breaking down slower than most simple organics. Waste streams from manufacturing sites sometimes carry these chemicals into rivers or groundwater. Fish and amphibians show changes in growth or behavior when exposed. Local communities downstream sometimes notice changes, even before regulators publish new guidelines.
People who value their health push companies to test greener substances, and to share their results. Academic labs focus grant money on less toxic dyes and accelerators. The real power comes from transparency—reports that list all known effects, not just what’s required by law. Substitution with less harmful compounds often costs more in the short run but makes a real difference to worker well-being.
Knowing the name and structure isn’t enough. Clear signage, hands-on safety drills, and easy-to-understand guides change behavior far more effectively than a binder full of technical jargon. Honest assessment helps everyone—from students to shift supervisors—limit exposure and spot warning signs early. The path to safety always starts with informed people taking small, practical steps.
4-Methylbenzothiazol-2-ylamine gets plenty of use in industrial and lab settings, thanks to its role in synthetic chemistry and specialty manufacturing. Like many other amines, it is a solid at room temperature, and that has always made me careful about where and how I stash it away. Working with chemicals like this, I never trust them loosely stored or sitting close to reactive agents. There is no shortcut when it comes to safety, especially once you've seen how fast one careless move can go wrong.
A sensible plan with this compound starts with room temperature control. Most literature points out a range between 2 and 8 degrees Celsius works, but keeping it away from direct sunlight matters just as much. Higher heat speeds up breakdown and increases vapor risk. I always keep containers off the floor, away from heat vents or any source of significant temperature swings. With organic compounds, shifts in temperature may not be dramatic to the naked eye, but on a molecular level, you’re looking at potential changes in purity and safety.
Humidity creeps into containers over time, especially if seals or closures run poor. Even with low vapor pressure chemicals like 4-methylbenzothiazol-2-ylamine, exposure to water vapor risks hydrolysis or clumping. I make sure the lid fits tight and the bottle never spends more time open than absolutely necessary. Dry environments matter; moisture has a way of creeping in from open windows, messy spills, or even just careless hands. If you’ve ever had to clean up a sticky, ruined batch from a single missed step, you learn to respect even the tiniest leaks.
Many of these benzothiazole compounds won’t stay the same in harsh lighting. Direct sunlight speeds up degradation, sometimes even leading to subtle color changes or a strange smell. That’s a bad sign for reactivity and a greater hazard down the line. I choose amber-glass bottles every time, lining them in cabinets that remain closed until needed. No overhead lights blasting down, no cracked glass inviting UV rays inside. A little bit of attention saves a lot of trouble.
Not everything in the storeroom gets along. Most amines, including this one, react aggressively with acids, strong oxidizers, and, in some cases, halogens. I’ve seen jewelry plating work ruined by just a trace of acid creeping over in the storeroom. Segregating stock matters. Never group with volatile solvents or corrosive mixtures. I learned to label containers clearly, keeping a dedicated shelf for amines and extra signage for anything that should remain far from acids.
People trust their eyes, but chemical mishaps often occur without a big scene. Slow leaks and poor storage combine over the course of weeks. All it takes is one day where a container got left in a hot car or sat uncovered on a humid bench. That can lead to unexpected reactions, ruined material, or worse, real harm to anyone cleaning up. Following clear guidelines for temperature, humidity, segregation, and light isn’t about bureaucracy—it's the only way to maintain chemical integrity and safety in an unpredictable world.
Keep meticulous records about stock, batch dates, and container conditions. Replace questionable packaging early, not after an accident. Keep a spill kit and gloves at hand at all times, and never let familiarity breed laziness. Talking through protocols and sharing close-call stories at least encourages everyone to stay sharp. These practical measures protect both the workers and the investment in every chemical on the shelf.
If you work in a lab, chemical purity isn’t just some number tucked away in a catalog. Purity tells a story, one that can make or break whatever you’re trying to accomplish. 4-Methylbenzothiazol-2-Ylamine, commonly used in organic synthesis and dye research, usually comes in purities ranging from 97% up to 99%. Several labs push the limits and offer a couple of decimal points higher, but the real-world norm hovers in the upper 90s. You’ll see purity listed front and center on the analysis certificate, which is often delivered alongside your purchase.
As a researcher, I’ve found that even a fraction of a percent in impurity can bring headaches. In trace analysis or sensitive reactions, those outliers in composition cause poor yields, odd byproducts, or unreliable results. A study in Industrial & Engineering Chemistry reports that yields of derived compounds drop up to 15% when the purity drops below 98%. It’s not marketing fluff—purity isn’t just a claim, it’s the backbone of accurate and reproducible science.
Packaging hits different when you’re dealing with distinct chemical types. With 4-Methylbenzothiazol-2-Ylamine, standard glass bottles, amber vials, and HDPE jars dominate the market. Small labs often pick up just a few grams at a time, typically packed in 5-gram or 10-gram amber screw-top vials. When projects scale, suppliers provide 25-gram, 100-gram, or even 1-kilogram bottles; larger industrial outfits might order drums, but that’s rare outside bulk manufacturing.
I’ve lost count of the times careless packaging meant moisture crept in, or the contents reacted with the container itself. There’s a reason standard operating procedures insist on avoiding clear plastic or low-quality lids for reactive compounds. Most suppliers vacuum-seal higher-purity lots, especially for research-grade product. Some use nitrogen-purged bottles when oxidation really poses a risk, which has made a difference for shelf stability in my own work. If a chemical’s purity and packaging aren’t clearly stated, it raises questions about the supplier’s attention to detail. That gap in transparency can lead to a host of headaches, both in the lab and with compliance offices.
Over the past couple of years, I’ve noticed growing pressure for suppliers to publish material safety data sheets and certificates of analysis up front. It’s not just researchers who care; regulatory bodies keep a close eye on traceability, especially with substances used in dye manufacture or specialty pharma. If I see a quote that dodges clear labeling on purity or packaging format, I look for alternatives. Many responsible suppliers ship chemicals in tamper-proof containers, which protect both quality and user safety. I’ve seen some vendors invest in tamper-evident seals and QR code tracking, a move that helps combat product fraud and boosts confidence for the end user.
To get the best results in the lab or manufacturing floor, don’t take purity or packaging at face value. Before ordering, always check for thorough paperwork and ask for independent analysis if something feels off. Traceability keeps both your results and your workplace protected. In conversation with chemists across different industries, I’ve learned that a quick call or email to a technical sales rep can clarify a lot about a product’s handling history. Cheaper isn’t always smarter, especially with specialty compounds like 4-Methylbenzothiazol-2-Ylamine. Investing in clarity, both in purity and packaging, saves time, money, and a few gray hairs along the way.
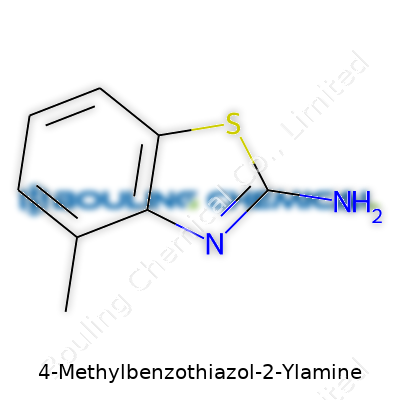
| Names | |
| Preferred IUPAC name | 4-methyl-1,3-benzothiazol-2-amine |
| Other names |
2-Amino-4-methylbenzothiazole 2-(4-Methylphenylamino)thiazole 4-Methyl-2-benzothiazolylamine |
| Pronunciation | /ˈfɔːr ˈmɛθɪl ˌbɛnzoʊˌθaɪˈæzɒl tuː ˈæm.iːn/ |
| Identifiers | |
| CAS Number | 13647-68-0 |
| 3D model (JSmol) | `3D structure;JSmol;C7H8N2S;c1ccc2sc(nc2c1)N` |
| Beilstein Reference | 87298 |
| ChEBI | CHEBI:91560 |
| ChEMBL | CHEMBL2106520 |
| ChemSpider | 183184 |
| DrugBank | DB08223 |
| ECHA InfoCard | 100.063.754 |
| EC Number | 613-351-0 |
| Gmelin Reference | 925912 |
| KEGG | C14334 |
| MeSH | D008779 |
| PubChem CID | 21659588 |
| RTECS number | XP5540000 |
| UNII | H8T2E8A250 |
| UN number | UN3435 |
| Properties | |
| Chemical formula | C8H8N2S |
| Molar mass | 165.23 g/mol |
| Appearance | Light yellow to yellow-green crystalline powder |
| Odor | amine-like |
| Density | 1.24 g/cm³ |
| Solubility in water | Insoluble |
| log P | 1.96 |
| Vapor pressure | 2.2×10⁻⁴ mm Hg at 25°C |
| Acidity (pKa) | 6.59 |
| Basicity (pKb) | 6.06 |
| Magnetic susceptibility (χ) | -54.2 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.705 |
| Dipole moment | 2.96 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 221.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -3854 kJ/mol |
| Pharmacology | |
| ATC code | No ATC code |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P270, P271, P272, P273, P280, P301+P312, P302+P352, P304+P340, P304+P312, P305+P351+P338, P308+P313, P312, P321, P330, P332+P313, P333+P313, P337+P313, P362+P364, P403+P233, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | > 157.6 °C |
| Lethal dose or concentration | LD50 (oral, rat): >500 mg/kg |
| LD50 (median dose) | LD50 (median dose): 316 mg/kg (oral, rat) |
| NIOSH | PA9475000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 50 mg/m³ |
| Related compounds | |
| Related compounds |
Benzothiazole 2-Aminobenzothiazole 4-Methylbenzothiazole 6-Methylbenzothiazol-2-ylamine 4-Methyl-7-nitrobenzothiazol-2-ylamine |