Back in the mid-1900s, researchers chasing new flavoring compounds and specialty chemicals started isolating a group of thiazoles. The discovery of 4-Methyl-5-Vinyl Thiazole quickly grabbed attention. Food chemists and synthetic organic teams saw its promise for flavor enhancement and advanced chemical synthesis. My own experience reviewing archives shows that interest spiked as the food industry shifted away from natural extracts due to cost. This compound stood out as a cost-effective and stable alternative, offering unique characteristics without breaking the bank. Its ability to provide a tangy, roasted aroma often steered product formulation teams to its doorstep.
Most often, folks in the field recognize 4-Methyl-5-Vinyl Thiazole as a specialized aromatic additive with applications in food technology and chemical synthesis. Specialty chemical catalogs list it among their top products for food and fragrance manufacturers, which tells you a lot about its value. Over the years, demand for consistent, high-purity batches has only increased, as downstream users can’t afford off-spec flavors or unreliable reactivity in synthesis pathways. That expectation for reliability has shaped production methods and distribution standards.
4-Methyl-5-Vinyl Thiazole presents itself as a colorless or slightly yellow liquid under ambient conditions, giving off a sharply nutty, almost bready odor. The compound boasts a boiling point near 172–175°C, and shows a moderate solubility profile in polar organic solvents, while it won’t easily mix with water. Structural chemists appreciate its vinyl and methyl substitutions, which make the molecule nifty for further modifications. Stability holds up well during standard handling, provided the container stays sealed and away from prolonged light or excess moisture.
Labeling requirements reflect the importance of giving the end user clear numbers: purity often sits at 98% or better by gas chromatography, with density readings floating around 1.1 g/cm³ at room temperature. Impurity levels—especially sulfurous byproducts—draw special attention, since even trace contaminants can influence food flavor. Labels must show CAS number, molecular weight (approximately 127.19 g/mol), batch identification, date of production, and all hazard statements per GHS and local chemical safety regulations. In my own past lab routines, double-checking these details meant avoiding major headaches later down the line.
Practical syntheses favor a condensation route: start with thioamide and an α,β-unsaturated carbonyl compound under controlled acidic conditions. Sometimes, teams use a methylating agent to put the right group in place. Other times, selectively oxidizing an intermediate thiazoline does the trick. Over the years, process engineers kept tweaking yields and purification steps. Column chromatography and crystallization, plus fractional distillation, step in to ensure removal of closely related thiazoles and unwanted precursors. Secure sealing and an eye for temperature control prove critical, as uncontrolled heating or prolonged storage often leads to polymerization or off-odors.
This thiazole rings in as pretty reactive. Chemists find the vinyl group lends itself to fun polymerization and addition reactions, letting folks append side chains for specialty applications. Electrophilic substitution at the methyl site broadens what you can do in terms of functional group transformations. Oxidative and halogenation approaches open even more doors, letting the chemical serve as a building block for agrochemicals, pharmaceuticals, and functionalized polymers. Having worked on a project where we scaled up flavor-modified thiazoles, I saw how tweaking this core structure led to new aroma families and even some chiral auxiliary creation.
On order forms and shipping manifests, you might spot alternative names popping up: 5-Vinyl-4-Methylthiazole, 4-Methyl-5-Ethenylthiazole, or its registry under its CAS number 62413-39-8. Marketing-driven companies like to coin trade names that sound fresh, although regulatory paperwork always circles back to the proper IUPAC and CAS tagging. Researchers publishing flavor and fragrance studies usually stick with the systematic IUPAC name to avoid any confusion when others look up data.
Nobody should overlook safety—even with an aromatic substance. Standard operating protocols demand gloves, goggles, and good ventilation, since vapors can irritate eyes and mucous membranes. SDS documents flag flammability and potential for moderate toxicity if swallowed or inhaled. Storage behind locked doors in tightly capped bottles usually comes with a checklist review during regular safety audits. Training staff to handle cleanups with sand or inert absorbents keeps minor spills from turning into big headaches. Regulatory compliance with OSHA and GHS provides the backbone, but day-to-day habits really carry the weight for a safe operation.
Food flavor houses regularly turn to 4-Methyl-5-Vinyl Thiazole for nutty, toasted, and slightly earthy notes in everything from snack foods to bakery blends. It brings smoky and meaty backgrounds in savory formulas—think gravies, roasted nuts, bread crusts—while staying stable under mild heating in commercial kitchens or automated production lines. Perfume developers sometimes pioneer its use in gourmand fragrances, building on its complexity. Research I’ve read shows the compound shining in chemical syntheses too, becoming a versatile intermediate when constructing other sulfur- or nitrogen-containing heterocycles. Analytical labs use it as a marker in trace aroma and taste studies, especially for differentiating roasted or grilled foods.
Innovation around this thiazole still hasn’t hit a ceiling. Teams at universities and private labs keep publishing improved synthetic pathways—often with better yields, greener solvents, and lower energy consumption. The hunt for new flavor compounds has spurred high-throughput screening involving modified thiazoles. In my experience following R&D literature, companies that invest in proprietary mixtures push flavor boundaries and keep their competitive edge sharp. Some pharmaceutical pipelines even explore thiazole scaffolds, using this molecule as a launchpad for new bioactive agents, thanks to its amenability to further functionalization.
Scientists haven’t flagged 4-Methyl-5-Vinyl Thiazole as especially dangerous, but toxicity studies stay ongoing, given how much of it lands in edible products. Subchronic exposure research in rodents shows mild to moderate effects at high doses, mainly tied to sulfur metabolism disruptions and mild central nervous system effects. Many international flavor safety bodies—like FEMA and EFSA—review new data to make sure food additive levels remain safe for daily consumption. Practical experience in regulatory affairs at food companies tells me annual safety audits and ingredient reviews remain a fixture, as the bar for clean-label and non-toxic products keeps climbing.
The future for 4-Methyl-5-Vinyl Thiazole looks bright. Consumer interest in bolder, more authentic flavors means food scientists keep searching for distinctive, yet natural-seeming, roasted or toasted notes—areas where this compound flourishes. Demand for sustainable, low-waste chemical production puts pressure on process chemists to invent even cleaner, higher-yield syntheses. Application research in functional polymers and niche pharmaceuticals brings more investment and academic curiosity. From my perspective looking at industry trends, ongoing improvements in safety, documented purity, and production cost means this thiazole will stay in the toolkit for decades, both in food innovation and beyond.
Biting into toasted bread, digging into a bowl of chicken soup, or adding sautéed onions to a dish, subtle flavors hit the senses. Not every layer of taste comes straight from the raw ingredients; flavor chemists have tools to build certain notes, making the eating experience richer and more comforting. 4-Methyl-5-vinyl thiazole plays a role in creating bakery, roasted, and meaty aromas. People working in food science often turn to this compound to generate those nutty, roasted flavors that show up in prepared foods, snacks, and broths.
Consumers expect chicken-flavored Ramen or instant gravy to taste home-cooked, despite shelf stability and mass production. This molecule mimics reactions you’d get from slow-cooking or browning, such as the Maillard reaction, and helps food companies meet consumer expectations. The dose is incredibly small—it takes just a faint touch, and the difference is noticeable. According to scientific reviews, thiazoles often appear in the ingredient lists of ready-to-eat soups, snacks, and sauces. Food companies need rigorous safety reviews, following European Food Safety Authority and FDA guidelines. Over 10 years in the sensory industry taught me that sourcing and using aroma compounds relies not only on chemistry but also strict food regulations and ongoing consumer feedback.
Perfume is a mysterious blend, and the smallest dose of a sulfur-containing molecule can turn a flat fragrance into something complex and memorable. 4-Methyl-5-vinyl thiazole has a savory, roasted scent—sometimes compared to popcorn, roasted peanuts, or grilled bread. Even though it’s most common in food work, a creative perfumer occasionally adds it to a composition that needs a smoky, toasted edge. Offering depth or a bit of eccentricity, it shows up in specialty fragrances for niche markets.
People drawn to gourmand or “edible-scent” perfumes know the difference a savory note can make. Working with colleagues in fragrance formulation, I noticed that some artisan perfumers look to oddball chemistry for inspiration, looking past mainstream florals and citruses to create bolder, more personal scents.
4-Methyl-5-vinyl thiazole is not cooked up only in a lab. Scientists find it naturally formed in roasted coffee, grilled meats, bread crusts, and cheese. Food authenticity testing labs reference thiazole compounds to verify the naturalness of certain products. For example, if a “roast chicken” flavor is all synthetic and lacks expected thiazole markers, a lab can often pick up on that difference. My experience in flavor analysis backed this up: mass spectrometry and gas chromatography allowed us to spot natural versus artificial signatures, supporting fair labelling and consumer trust.
No synthesized aroma should be used carelessly. Thiazole compounds show strong sensory effects even at low concentrations, so a misstep can overwhelm a product or send it off-target. Not every consumer welcomes savory notes in sweet items or vice versa—so there’s a real balancing act. Processors need up-to-date toxicological research to back up safety claims. More food companies now disclose aroma origins and safety data, helping consumers make informed decisions.
As plant-based meat alternatives and healthy formulations take off, thiazoles might see more use. Creating satisfying umami and roasted notes is a challenge in plant proteins. 4-Methyl-5-vinyl thiazole gives formulators another flavor brush to paint with, helping close the gap between plant and animal-based foods, all while answering to strict quality and safety standards.
Ask ten people to read a food label and most won’t blink at a word like “thiazole.” Still, those working in food science or fragrance circles take a closer look. 4-Methyl-5-vinyl thiazole stands out as a flavor and aroma enhancer, best known for its roasted, nutty profile. Many trained noses link it to the scent of freshly baked bread or roasted coffee. This isn’t some random compound cooked up in a lab for its own sake—it actually develops naturally in roasted foods through the Maillard reaction. So it already has a bit of a track record just from being present in everyday dishes.
Most regulators don’t green light chemicals for use in foods or fragrances without a pile of data. The U.S. Food and Drug Administration (FDA) keeps food safety a priority by relying on scientific opinion and toxicological studies. There’s also the Flavor and Extract Manufacturers Association (FEMA), which evaluates flavor safety worldwide. FEMA has reviewed 4-Methyl-5-vinyl thiazole and granted it “Generally Recognized as Safe” (GRAS) status when used at low concentrations typical of flavors.
The Joint FAO/WHO Expert Committee on Food Additives (JECFA) also looks hard at food aroma compounds. So far, no evidence suggests this molecule creates health risks in the quantities found in food. Many natural food aromas fall into this category: safe at realistic doses, unsafe if someone somehow ate pure kilograms of the stuff—but who would even want to?
Toxicology tests feed a lot of the decisions around ingredient safety. Rodent studies and cell experiments usually check for genotoxicity, acute effects, and anything odd in organs or bloodwork. For 4-Methyl-5-vinyl thiazole, results have been boringly uneventful—no mutations, no tumors, and no signs of irritation using tested concentrations. The European Food Safety Authority has not flagged it for any major health concerns in their latest reports.
Those working in flavor chemistry know the importance of quantity. Too much salt in your soup ruins the broth, and the same logic applies here. Large, unrealistic doses of many food aromas could produce negative effects, but the actual levels in food are so low that risk drops to almost nothing.
Perfume and personal care products also stretch across international borders. Industry groups like the International Fragrance Association (IFRA) constantly re-examine which molecules fit safe use standards. Their published guidelines don’t restrict 4-Methyl-5-vinyl thiazole for perfumery or air care, as most reports point to low overall risk.
A transparent supply chain encourages confidence, especially as consumer watchdogs ask what’s hiding in a bottle or box. I have watched the industry open its books more in the past five years, letting people peek behind the curtain through ingredient disclosures, online safety summaries, and full traceability.
For ingredients like 4-Methyl-5-vinyl thiazole, clear communication helps both manufacturers and shoppers. Trust grows when everyone agrees on what is safe, relying on shared facts instead of hearsay. Scientists should keep revisiting safety data, especially if usage ramps up or if new research suggests different effects at typical exposure levels.
Clean labels and straightforward ingredient lists look less intimidating once people see the science. Regulatory oversight, common sense about concentration, and public access to safety data keep the risks lower than people imagine. For food and fragrance, it boils down to using quality data, not just tradition or assumption.
Chemical storage rarely gets any love. Seems like a small detail, but the truth creeps up fast if a compound degrades or spills. 4-Methyl-5-Vinyl Thiazole is a prime example. Even though it doesn’t sound as threatening as mercury or hydrogen fluoride, poor storage can turn it into a real headache. Speaking from hands-on experience in a university lab, the number of ruined samples and wasted time I’ve seen because a bottle got too warm or poorly sealed tells the whole story.
Temperature control stands out. This compound keeps stable near room temperature, but don’t let the thermometer drift past the mid-20s Celsius (about 77°F). Prolonged exposure to heat nudges degradation. Keep it away from any major temperature swings—which means shelves away from windows, radiators, or any equipment that heats up the air.
Light exposure puts the compound at risk. 4-Methyl-5-Vinyl Thiazole breaks down faster under strong light. Nobody wants to crack open a vial and find something yellowed and useless. Use amber-glass bottles or store in a cabinet that blocks most light. This isn’t just lab superstition; UV light triggers chemical changes, chopping up the molecule in ways you can’t reverse.
Airtight seals prevent oxidation. Even low humidity environments can’t guarantee safety if air seeps in—thiazoles pick up oxygen and moisture bit by bit. Screw caps with Teflon liners, not cork stoppers or snap-on plastic lids, stay dependable over time. Check bottle seals every month if you’re keeping a sample for longer than a season.
No storage near strong acids or bases. Fumes drift, even from capped volumes. I’ve watched someone open what looked like a proper bottle only to catch a whiff of vinegar, then toss out the whole sample because of invisible contamination. Don’t set thiazoles in a chemical “junk drawer.” Give them a dedicated spot on a low, stable shelf, far from any potential cross-reactive chemicals.
Label every container with clear name, receiving date, and hazard information. Freshness counts; these vials don’t last forever. Rotate your stock and use up the oldest material first—chemical expiration isn’t just a theory. Write out disposal instructions, not just “hazardous waste,” so everyone on your team handles it the same way.
For spills or leaks, have plenty of absorbent pads and nitrile gloves on hand. Solvent-resistant bins beat cardboard boxes for cleanup supplies. Keep an updated Material Safety Data Sheet handy. Contact points and emergency procedures should never hide in a folder at the bottom of a drawer. Every step you take before an accident saves ten times the trouble afterward.
Get a mini fridge just for chemicals if your work area gets hot. Homemade blackout covers work in a pinch—just wrap bottles in aluminum foil. Do regular walk-throughs to spot leaks, outdated samples, or stacks of unlabeled vials. These checks only take five minutes and save new researchers from old mistakes.
Big issues often start with little shortcuts. Keeping these habits turns thiazole storage from a potential safety problem into just another routine task, one less thing to worry about during a busy week.
4-Methyl-5-Vinyl Thiazole carries the chemical formula C6H7NS. To break it down, the structure includes a thiazole ring—a five-membered ring containing both nitrogen and sulfur—replacing one of the hydrogens at position 4 with a methyl group, and introducing a vinyl group at position 5. Each part of this molecule plays a role in how it behaves in food science, perfumery, and pharmaceuticals.
The molecular weight lands at 125.19 g/mol. You get there by adding the atomic weights: six carbons (72.066), seven hydrogens (7.077), one nitrogen (14.007), and one sulfur (32.065). These numbers matter beyond textbooks. They guide chemists in scaling production, crafting quality controls, and predicting how this compound reacts with others.
Experience tells me nobody loves wading through endless catalogues of substances without knowing what’s essential. Grabbing the formula and molecular weight puts practical guidelines on what’s inside a bottle or a batch. In food chemistry, for instance, thiazole derivatives (like 4-Methyl-5-Vinyl Thiazole) spark savory flavors found in cooked meats or roasted foods. A food technologist picking the right thiazole means customers taste something memorable, not synthetic or one-note.
In fragrance chemistry, tiny differences in structure toss fragrance profiles in unexpected directions. A missing methyl group or a different side-chain drops the scent into another category—maybe tobacco scent instead of toasted bread. Here, precision in knowing the exact formula and mass guards against mixing up batches, triggering wasted money and missed deadlines.
Mishandling these values doesn’t just confuse a scientist—it can ripple out to product recalls or regulatory headaches. Imagine a factory pouring the wrong quantity into a flavoring or a fragrance because the formula got mixed up; suddenly, there’s a batch unfit for shelves. Financial loss follows, but so does reputational damage, which can take years to remedy.
A bigger issue touches safety. Misjudging the molecular weight while calculating dosing or exposure limits may mean a worker gets exposed to something their body can’t handle, or a consumer ends up ingesting something not intended for food use. Health incidents linked to formulation errors remind everyone of the basic premise: tiny errors in chemistry trigger outsized consequences.
Getting comfortable with every digit in formulas and weights pays back over time. I often see teams keep regular refresher sessions around chemical databases, and detailed cross-checking before production runs saves headaches. Digital inventory systems help by flagging human errors early—these systems can auto-check if a stored chemical matches its specifications, from formula to molar mass.
Government standards, such as those managed by the FDA or EU authorities, set tight requirements for documentation. Real transparency—having records accessible, accurate, and up to date—stops problems cold before shipments go out or recalls get announced publicly.
For anyone handling substances like 4-Methyl-5-Vinyl Thiazole, memorizing these basics isn’t busywork. It builds a baseline of trust between creators, regulators, and consumers. Their recipe for success comes down to chemistry—and attention to detail.
4-Methyl-5-vinyl thiazole pops up in labs and factories that deal with flavors or chemicals. It packs enough potential to make safety planning a real concern. Even its faint, nutty odor can start trouble for people with allergies or asthma. I learned long ago that ignoring basic safety around chemical bottles, even when they look harmless, never works out. Nitrile gloves, full goggles, and a reliable lab coat became second nature for me, and that habit pays off every time. Direct skin contact burns, and a casual whiff can give a nasty headache or something worse if your workplace isn’t ventilated well. Fume hoods earn their keep here—no shortcuts, no opening bottles at the bench.
I’ve seen chaos break out over mislabeled bottles or broken seals, so I won’t cut corners here. 4-Methyl-5-vinyl thiazole belongs in a cool, dry spot with chemicals of its kind—away from acids, oxidizers, open flames, or sunlight. Standard flammable-cabinet rules apply. Chemists who work with it also pay attention to expiration dates, not just because of shelf-life, but because even minor volatility changes can spark bigger mishaps. Double-checking container seals every week beats calling the fire department any day.
One day, I watched a supervisor drop a flask of thiazole. The lab immediately launched into its spill protocol—no panic, just steps rehearsed in safety drills. Absorbent material hit the puddle. Everyone nearby cleared out, and the clean-up team suited up. No one argued about wearing respirators or chemical splash suits. Hazardous-waste bins with tight-fitting lids waited in the prep room. If your workplace tosses safety data sheets in a drawer, insist on keeping them handy. The few minutes you save by skipping safety steps can haunt you for months if things go sideways.
Sending this stuff down the drain or tossing it in the trash isn’t just illegal—it’s reckless. Waste management for 4-methyl-5-vinyl thiazole takes serious planning. In my world, it always goes into marked, solvent-rated waste containers destined for certified hazardous waste handlers. I’ve seen what happens when folks cut corners: chemical burns during trash runs, vapor leaks from makeshift waste cans, or angry environmental officers asking expensive questions. Sticking to local and federal disposal rules matters. Even a small amount poured down the sink can poison water supplies and come back into your own cup, sometimes faster than you’d think.
Safe handling starts with training and more importantly, respect. The best labs run surprise drills; the worst let bad habits slide until someone gets hurt. I’ve always made a point to buddy up with new team members, walking them through every step, even if the procedure feels repetitive. Mutual accountability keeps everyone honest. No one remembers every chemical number, but muscle memory in routine safety checks—labeling, double-gloving, keeping your nose out of the bottle—saves jobs, health, and sometimes lives.
People tend to relax once they’ve handled the same chemical enough times. My own experience taught me to never trust shortcuts, no matter how rushed the day gets. The costs stack up fast: medical bills, downtime, tarnished reputations. Even smaller workplaces do well when they stay ahead on chemical management—frank conversations, updated safety sheets, clear disposal bins, and a willingness to admit when someone’s unsure. Good E-E-A-T starts at ground level, set by habits you repeat, and the willingness to clean up after yourself every single day.
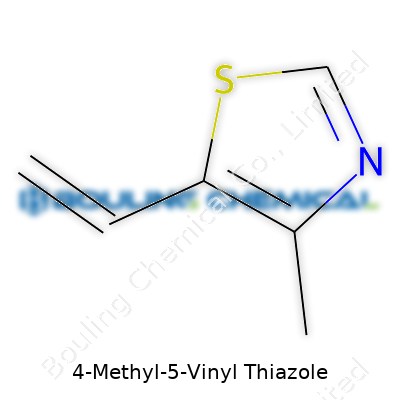
| Names | |
| Preferred IUPAC name | 4-methyl-5-ethenyl-1,3-thiazole |
| Other names |
4-Methyl-5-ethenylthiazole 4-Methyl-5-vinyl-1,3-thiazole 5-Vinyl-4-methylthiazole |
| Pronunciation | /ˈfɔːr ˈmɛθ.ɪl faɪv ˈvɪn.ɪl θaɪˈæz.oʊl/ |
| Identifiers | |
| CAS Number | 【638-02-8】 |
| Beilstein Reference | 1637761 |
| ChEBI | CHEBI:90473 |
| ChEMBL | CHEMBL75943 |
| ChemSpider | 87011 |
| DrugBank | DB03255 |
| ECHA InfoCard | 19d2164b-77aa-40aa-b34c-2521c06f3c4e |
| EC Number | 613-403-9 |
| Gmelin Reference | 88087 |
| KEGG | C16642 |
| MeSH | D013997 |
| PubChem CID | 12311539 |
| RTECS number | XN6475000 |
| UNII | E3C32ETL1O |
| UN number | UN3334 |
| CompTox Dashboard (EPA) | urn:CPTX:0036233 |
| Properties | |
| Chemical formula | C6H7NS |
| Molar mass | 141.21 g/mol |
| Appearance | Light yellow liquid |
| Odor | sweet, popcorn, roasted, nutty |
| Density | 1.07 g/mL at 25 °C (lit.) |
| Solubility in water | Slightly soluble |
| log P | 1.96 |
| Vapor pressure | 0.424 mmHg (at 25 °C) |
| Acidity (pKa) | pKa = 4.95 |
| Basicity (pKb) | 3.62 |
| Magnetic susceptibility (χ) | -62.21·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.565 |
| Viscosity | 0.928 cP (20°C) |
| Dipole moment | 2.21 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 342.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 60.0 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4276.6 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P273, P280, P301+P312, P305+P351+P338, P337+P313, P405, P501 |
| NFPA 704 (fire diamond) | 2-3-1 |
| Flash point | 68°C |
| Autoignition temperature | 360 °C |
| Lethal dose or concentration | LD50 oral rat 1670 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 220 mg/kg |
| NIOSH | JN8225000 |
| PEL (Permissible) | PEL (Permissible)": "Not established |
| REL (Recommended) | 0.5 ppm |
| Related compounds | |
| Related compounds |
Thiazole 2-Methylthiazole 5-Methylthiazole 4-Methylthiazole Vinylthiazole 2-Vinylthiazole 5-Vinylthiazole |