Interest in thiazole derivatives stretches back to the beginnings of organic chemistry in the late 19th century, when German and French chemists mapped out heterocyclic rings and their properties. Thiazoles became especially important once the pharmaceutical world discovered their presence in antibiotics like penicillin. 4-Methyl-5-formyl thiazole, in particular, hit a stride when scientists began searching for analogues to natural thiazole-containing compounds, hoping to bolster antibacterial or flavoring agents. In the mid-20th century, researchers learned to synthesize new variants more reliably, and by the 1970s this compound found a role as a key intermediate in both pharmaceutical synthesis and flavor research. This history tracks not just a molecule, but the stubborn, creative hunt for compounds that bridge ideas between biology, chemistry, and industrial application.
4-Methyl-5-formyl thiazole comes off as a modest yellowish liquid, sporting a sharp, nutty odor. Daily use rarely brings most people into contact with it in pure form, yet the compound’s presence is felt throughout industries that touch on flavors, pharmaceuticals, and specialty chemicals. Researchers value it as a building block for synthesizing heterocyclic compounds, with many laboratories storing it alongside other thiazole derivatives. Its packaged form often appears in amber glass bottles, caution labels where required, and batch numbers kept for traceability in regulated markets.
You’ll find 4-methyl-5-formyl thiazole described with numbers: molecular formula C5H5NOS and molecular weight just under 127 g/mol. Its boiling point lands between 200 and 220°C. That modest volatility sets it apart from lighter solvents, yet its strong aromatic odor remains noticeable even in low concentrations. Solubility skews toward organic solvents rather than water, playing into its role in non-polar reaction systems. Chemists who work with it grow familiar with the way its carbonyl group at the 5-position pushes the molecule toward further transformation during synthesis. Its refractive index sits between 1.57 and 1.60, a detail lab workers watch for in purity checks. Its yellow hue and low flash point add a practical warning for those handling it on the bench.
Specification sheets for 4-methyl-5-formyl thiazole leave little room for guesswork. Most manufacturers will quote assay values over 97% purity, outline allowed moisture content, and list storage conditions that dodge sunlight and high humidity. Bottles arrive tagged with UN numbers for transport, safety icons for eye and respiratory protection, and hazard statements for skin or inhalation exposure. On the analytical side, labs check NMR and IR spectra for structural consistency and run HPLC or GC-MS to ferret out impurities. FDA and EU flavoring regulations look for contaminants below threshold levels for food use; pharmaceutical grades face even stricter scrutiny. Each batch comes with a lot number, vital when regulatory agencies backtrack through a supply chain after any incident.
Laboratories have hammered out several ways to get to 4-methyl-5-formyl thiazole. One classic approach starts with thioamide and α-haloketone reactions, guided by the Hantzsch synthesis route first explained in the late 1800s. Today’s chemists often prefer more controlled one-pot syntheses, minimizing byproducts and purifying the target compound in fewer steps. Starting with 4-methylthiazole, oxidation with mild agents like selenium dioxide introduces the formyl group smoothly. Greener labs may swap out toxic oxidants for catalyzed air oxidations, looking to nudge yields up and keep waste down. Isolation usually involves distillation or silica gel chromatography, watching for that yellow fraction in column runs.
The aldehyde group at position 5 invites a wealth of paths for further chemistry. Reduction drops it to a methyl group, cycloadditions attach aromatic rings, and condensation with nucleophiles allows flexibility in building larger molecules. More than a handful of drugs, aroma chemicals, and agrochemicals use 4-methyl-5-formyl thiazole as the scaffold for attaching bioactive substitutes. In my own research, this compound showed up as a lynchpin in synthesizing advanced intermediates—condensing with amines, it points directly toward Schiff base analogues that trigger biological effects in test assays. Thiazole’s sulfur and nitrogen make it reactive enough for cross-coupling, yet enough of a challenge that process chemists keep tweaking how best to control side reactions.
Across chemical suppliers and journals, you’ll see 4-methyl-5-formyl thiazole go under alternate names: 4-methylthiazole-5-carbaldehyde, 5-formyl-4-methylthiazole, and MFT for short. Some flavor companies reference it as a nutty or roast flavorant, weaving it into complicated product codes rather than spelling out the name. In regulatory filings, the IUPAC name rides along with a CAS number—5368-64-1—for unambiguous tracking.
Nobody who handles 4-methyl-5-formyl thiazole on a daily basis treats it casually. Direct skin contact brings irritation and a rash in sensitive individuals, and vapors build in closed spaces, leading to coughing, headaches, and in bad scenarios, dizziness. Standard procedure calls for gloves, goggles, and dedicated fume hoods. Spills get contained with inert absorbents, with waste bottles labeled for hazardous organics. Environmental release policies keep the compound away from drains and landfills. Exposure levels must line up with OSHA and EU workplace standards; labs use air monitoring badges and proper ventilations systems in spaces that store the chemical in bulk.
4-Methyl-5-formyl thiazole has found a living role across several industries, not just in academic labs. Food and fragrance creators add trace amounts to roasted, savory, or nutty aroma formulas. Pharmaceutical companies work it into synthetic routes for developing antifungal, antibacterial, or anticancer molecules, since the thiazole core shows up so often in medicines. In my own experience, its reactivity made it valuable in molecular library synthesis, letting researchers quickly scan for new drug candidates in high-throughput labs. Some crop protection agents use thiazole rings to inhibit pest enzymes, making this molecule a part of pushes to create more effective, targeted agrochemicals. Its unmistakable odor helps in flavor calibration for crackers, coffee, and roasted nuts—hitting that familiar “toasted” note.
Research teams keep wrestling with how to coax new properties out of thiazole derivatives. Synthetic chemists focus efforts on more sustainable oxidation and condensation strategies that lift yield and safety without raising costs. Biochemists explore how the molecule’s reactive aldehyde acts in cells, trying to design prodrugs or tracking labels that snap onto biomolecules in vivo. In the last decade, several papers have detailed catalyzed cross-coupling that graft functional groups to either the 4- or 5-position, expanding medicinal chemistry toolkits. Even the food sector keeps pushing to craft analogues that imitate or boost natural flavors. Funding tends to follow applications, and those who can prove a new route brings down waste, boosts yield, or opens an unexpected use receive more backing.
Studies in animal models show 4-methyl-5-formyl thiazole has moderate toxicity at high doses, with the aldehyde moiety linking to oxidative stress in liver tissues. Chronic exposure brings risks, though data remain sparse on long-term, low-level human ingestion. Industrial safety panels update worker guidelines as more data climb in. The European Food Safety Authority limits food additive use to fractions of a part per million, based on rodent and in vitro studies. Researchers keep evaluating metabolites to see if breakdown products linger or harm gut flora. Chemists keep close watch, and regulatory filings often piggyback off broader thiazole safety profiles until focused long-term studies draw more concrete conclusions.
Looking forward, the applications for 4-methyl-5-formyl thiazole will likely keep branching out. The surge in artificial intelligence for flavor design brings as much attention to subtle molecules like this as to mainstream esters and alcohols. Pharma pipeline advances depend on tweaking scaffolds and exploring untested chemical space; here, thiazole has yet to finish surprising anyone. Green chemistry trends coax suppliers to invent cleaner, faster ways to produce the compound. Food regulatory agencies tighten oversight and push for deeper studies of chronic low-dose exposure. Academic and industry labs both value the mix of established history and ripe-for-innovation future this molecule carries. My own time spent in chemical development and flavor labs convinced me that advances rarely come from finding new elements, but from re-imagining uses for compounds like 4-methyl-5-formyl thiazole, each time the world asks for something safer, tastier, or more effective.
Seeing names like 4-Methyl-5-Formyl Thiazole takes me right back to my early days hunched over cramped lab benches, measuring tiny quantities of powder, and feeling the pressure to get every calculation dead on. People outside chemistry circles probably never talk about this compound, but behind the scenes, it plays a role that deserves some attention. Anyone who’s worked making flavors or studying vitamins will tell you: some of the weirdest ingredients become vital to how food and nutrition shape up.
Every time I bite into toasted bread or sample a fresh tomato sauce, I think about the molecules behind those aromas. 4-Methyl-5-Formyl Thiazole belongs to the family of thiazoles, a type of compound that matters a lot in food science. This ingredient supplies a roasted, almost nutty note, sort of like the deep smells you get from browned onions or well-crisped crust. Flavorists rely on it to bring depth and complexity to processed foods, seasoning blends, and bakery goods.
Lab-produced flavors aren’t just about making artificial versions of natural tastes. They help with consistency, safety, and accessibility. Harvests fluctuate, prices go up and down, and sometimes real ingredients are in short supply. Engineers and chemists use thiazoles to fill the gap, sometimes even strengthening existing flavors. According to the FDA and EFSA, several thiazole derivatives have recognition as safe for use in food products as long as manufacturers follow established guidelines.
The chemistry world knows 4-Methyl-5-Formyl Thiazole for more than its flavor. During my university days, I remember research groups looking into thiazole rings because they form part of vitamin B1—thiamine. Without thiamine, bodies break down, and nerves misfire. That thiazole backbone you find in 4-Methyl-5-Formyl Thiazole shows up in a lot of drug discovery projects. Some scientists try tweaking the core to develop new antibiotics or drugs for neurological conditions.In pharmaceutical synthesis, variations of this molecule act as building blocks. They provide a structural base to build more complex therapeutics. Researchers use similar compounds to screen for new enzyme inhibitors, antifungal compounds, or even candidates for cancer therapy. Having that chemical flexibility lets scientists adjust features to suit different medical targets.
No commentary would be honest without tackling questions about safety. Some folks hear about chemicals in food or medicine and worry. That’s natural—I do it too, reading labels or digging through clinical studies. The key is clear data and transparency. Agencies like the Joint FAO/WHO Expert Committee on Food Additives monitor and publish findings on molecules used in these industries. Toxicology reports on thiazoles provide reassurance, provided dosage stays within tightly controlled limits set by regulations.
What matters for public trust comes down to evidence and open communication. I've seen skeptics change their stance after walking through the process: audits, regular reviews, and strict adherence to global standards.
Innovation in food and pharma doesn’t slow down. Researchers look for improved versions of thiazole compounds, hoping some minor tweak unlocks better shelf life, stronger medicinal properties, or a cleaner label. Companies working with these chemicals need to invest in updated safety testing and keep lines of communication open for consumers and regulators alike.
4-Methyl-5-Formyl Thiazole might not get a lot of headlines, but its quiet influence reminds us how chemistry shapes daily life. Knowing where and why we use these compounds helps consumers and scientists alike make smarter choices.
4-Methyl-5-formyl thiazole catches the attention of anyone who cares about chemistry’s impact in daily life. Thiazole compounds show up in pharmaceuticals, flavorings, and research labs for good reason. 4-Methyl-5-formyl thiazole stands out as a representative member of this group, with its own set of quirks in both its physical properties and its applications. Getting to the heart of its function means examining its structure top to bottom.
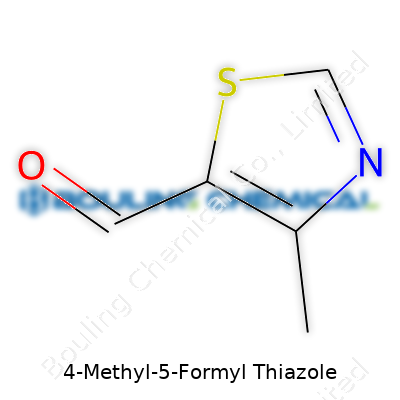
Start with the foundation. Thiazole itself sports a five-membered ring, fusing two kinds of atoms—sulfur and nitrogen—in a cycle with three carbons. The ring system appears deceptively simple, but what matters is what gets attached to it and where. The nitrogen and sulfur sit at distinct points: sulfur at position one and nitrogen at position three.
Taking thiazole and dressing it up with a methyl on the fourth carbon and a formyl (an aldehyde group, –CHO) on the fifth carbon gives you 4-methyl-5-formyl thiazole. Within the world of aroma chemistry, those tweaks turn a basic ring into a molecule that pops up in roasted, cooked, and warm flavor notes. Scientists analyzing roasted meat and coffee recognize this name when they parse through the volatile compounds responsible for taste and aroma.
The structure, written out, looks like this: the thiazole ring binds the methyl group at the fourth position—this carbon pulls in three hydrogens and connects to the ring through a single bond. At the fifth carbon, the ring holds a formyl group, defined by a carbon double-bonded to oxygen and single-bonded to hydrogen. That configuration introduces reactivity and gives the molecule distinctive chemical behavior compared to its unadorned parent ring.
Chemistry doesn’t exist in a vacuum. The special odor properties of 4-methyl-5-formyl thiazole stem from its structure. Food, beverage, and even fragrance industries constantly seek out these small molecules to improve profile and appeal. In the lab, the functional groups—methyl and formyl—open doors for derivatization, letting chemists build bigger, more complex molecules through simple chemical modifications.
An understanding of how small tweaks in structure drive changes in smell, taste, or reactivity brings value for pharmacists, toxicologists, and food technologists. For instance, traces of 4-methyl-5-formyl thiazole appear naturally in heated foods, while larger concentrations carry deeper implications for safety or health. Analytical chemists must rely on robust analysis and quality controls to keep limits in check.
Trustworthy data matter. According to PubChem and the Chemical Abstracts Service, the IUPAC name for 4-methyl-5-formyl thiazole is 4-methyl-1,3-thiazole-5-carbaldehyde. Its molecular formula breaks down to C5H5NOS, though that simple string doesn’t capture placement. Studies on odor thresholds in food show even trace amounts make a noticeable difference in flavor—showing how nuanced chemical structure acts on the senses.
Eyes on safety, lab protocols must focus on safe handling. Experience underscores that gloves, goggles, and fresh air win out every time. For those synthesizing or analyzing the molecule, understanding its functions—down to how it interacts with other chemicals—prevents mistakes and sharpens results. Tracing sources in food or environment can pinpoint ways to enhance quality, manage risk, and support clean labeling.
Designing substitutes and analogs with safer or more appealing profiles keeps research moving forward—offering better flavors or reducing unwanted byproducts. Pulling lessons from real-world chemical properties draws a clear connection between molecular structure and human experience.
Over the years working alongside researchers and lab staff, I’ve seen what can go right and wrong with storing specialty chemicals. 4-Methyl-5-Formyl Thiazole brings a mix of hazards—its formyl group makes it reactive and the thiazole ring can set off sensitivities. Ignoring storage guidance often leads to lost product, health risks, or even evacuation. Smart protocols don’t just follow the rules; they keep folks safe and protect investments in research.
A good start always includes using airtight containers made from materials that won’t break down or react with thiazoles. Glass or specific high-grade plastics do the job, but labeling means more than just writing a name. Every bottle or drum needs a clear hazard warning, date of arrival, and batch number—the details someone will need in an emergency or an audit.
Most labs I’ve seen keep sensitive compounds like this in small volumes. That limits waste if there’s a spill and helps avoid cross-contamination. If you’re opening and closing the bottle often, switching to smaller containers keeps the remainder pure and stable.
4-Methyl-5-Formyl Thiazole won’t do well in hot, humid, or direct sunlit rooms. Its chemical stability drops quickly once you break those boundaries. Aim for cool, dry storage—ideally a fridge or temperature-controlled cabinet set between 2°C and 8°C. Humid air and temperature swings kick off degradation or turn powders into sticky clumps, both of which ruin research or production runs.
A dry cabinet with desiccant packs will absorb stray moisture. Most experienced chemists toss a couple of fresh packs into storage spots and change them out during regular checks. I’ve watched entire batches saved from ruin that way, even during hot summer months.
Mixing storage of thiazoles with acids, oxidizers, or strong bases just adds risk. 4-Methyl-5-Formyl Thiazole can give off noxious gases or catch fire if it leaks into the wrong spot. Separate lockers or shelves make a huge difference. I’ve helped set up simple shelf maps with clear colored dots, so nobody grabs the wrong bottle or stores something next to bleach or nitric acid.
Carrying this compound between buildings or across a campus? Double-containment makes sense—one bottle inside a secondary shatterproof container lined with absorbent material stops accidents before they spread. Always move these bottles upright, and use trolleys or carts for anything heavier than a lunchbox.
In the lab, a chemical fume hood blocks exposure and vents irritant fumes. Nitrile gloves, lab coats, and proper goggles aren’t up for negotiation. After an exposure scare at a colleague’s bench, most teams made PPE second nature, rather than a box to check off.
For small spills, folks on my team used absorbent pads followed by a wipe-down with water and mild detergent, then bagged everything in hazardous waste bins. Large leaks meant everyone out and calling the site safety lead. Waste where 4-Methyl-5-Formyl Thiazole shows up—pipette tips, wipes, containers—all goes in labeled disposal drums for specialty chemical waste pick-up, never ordinary trash.
Documentation stays as important as safety glasses. Each batch in or out of the door gets logged, so nothing disappears, and it’s easy to spot patterns in waste or problems down the road.
Some folks see these steps as tedious, but the price of a shortcut always finds you later. Training up everyone, no matter their experience level, keeps labs running smoothly and people healthy. Regular reviews and restocking, along with a healthy respect for each chemical’s quirks, make the difference between a safe workplace and a disaster waiting to happen. It’s a practice rooted in years of witnessing how little mistakes can ripple out, costing time, money, and sometimes trust that safety rules work.
Science sometimes feels distant, but 4-Methyl-5-Formyl Thiazole isn’t a compound just for chemists in white coats. As someone who’s spent years following stories about synthetic chemicals in everything from cleaning products to food additives, learning about chemical safety isn’t something I take lightly. This thiazole derivative has popped up in labs, classrooms, and—for the extremely invested—various industry formulations. People studying chemical interactions or making flavor additives may recognize its name.
The safety of such chemicals means a lot when they play a role in research or process manufacturing. According to reliable safety data sheets and peer-reviewed research, 4-Methyl-5-Formyl Thiazole brings some cause for caution. Inhalation, skin contact, or accidental ingestion can cause irritation. This isn’t just theoretical: chemical catalog entries from major suppliers all flag the potential for side effects with direct exposure. Symptoms may look mild—a rash, a cough, maybe watering eyes—but the possibility for more serious outcomes exists, especially after prolonged or high-dose exposure.
What really sets off alarm bells comes from animal toxicity data. Laboratory tests suggest at high concentrations, this compound can create toxic effects, including damage to organs or biochemical imbalances. Even if human data isn’t as complete as some would want, responsible handling remains crucial, particularly for those mixing, measuring, or using it often. I’ve seen lab environments run into trouble by brushing off warning labels, leading to sick employees or investigations that shut down research for weeks.
Dealing with chemicals isn’t just a job for scientists. Many regular folks check food labels and ingredient lists. If a synthetic flavor or fragrance ingredient containing this compound shows up, transparency makes a difference. I remember the relief I felt when a knowledgeable pharmacist broke down risks versus benefits of a compound, compared to blank stares and evasive answers at a different store. That sense of trust starts with sharing clear information.
Information may sound dense, but what matters is knowing how exposure can affect your health. The National Institute for Occupational Safety and Health (NIOSH) highlights this same point: knowing the risks behind each step in handling or using a chemical, and making resources available to everyone using it. Even though 4-Methyl-5-Formyl Thiazole isn’t splashed across product shelves, any workplace or research lab using it needs clear protocol—gloves, safety goggles, ventilation. Ignoring good habits for the sake of speed or convenience leads to real accidents.
So, what can be done? Industry and labs must provide up-to-date safety sheets, train staff thoroughly, and keep spill response gear in plain sight. Regulatory bodies should step in, making sure companies publish health data and revisit chemical safety rules as new research comes to light. For consumers, pressing manufacturers to show their ingredient sourcing, and voting with your wallet, can drive a shift toward better clarity and safer use.
Learning about chemical safety means a lot more than memorizing scientific names. It’s about knowing the risks tied to each step, sharing what you learn, and keeping safety front and center for everyone—not just the professionals.
In the world of chemical research, purity isn’t just a buzzword — it’s a requirement for every experiment that aims for meaningful results. I’ve watched researchers pull their hair out over results ruined by barely-there contaminants. For a compound like 4-Methyl-5-Formyl Thiazole, demanded in fields from pharmaceuticals to advanced materials science, purity can make or break a project.
Impurities can change reaction outcomes, skew characterization data, and lead to safety problems nobody wants. That’s why everyone from graduate students to lead chemists puts so much emphasis on where and how they source chemicals.
Anyone on the hunt for 4-Methyl-5-Formyl Thiazole at the highest purity levels probably already knows the usual suspects. There are established scientific suppliers — well-known companies like Sigma-Aldrich (now part of MilliporeSigma), Alfa Aesar, and TCI America — who take transparency and regulatory compliance seriously. These suppliers demand proof of intended use, which supports safe handing across the supply chain.
Quality verification matters. I have questioned a certificate of analysis more than once, especially after an unexpected result. Sourcing from a reputable supplier eases those worries since they document their production and analytical processes. This focus on documentation protects not just the science but the people behind it.
There’s a temptation, especially under tight funding, to turn to little-known online sources or random “chemical marketplaces.” Offers might look attractive with low prices or free international shipping, but past experience tells me that risk outweighs reward. Aside from purity concerns, shipping hazardous chemicals across borders can mean legal trouble or lost shipments due to customs seizures.
Cutting corners on sourcing means gambling your experiment’s integrity. It costs more than money if a flawed batch ruins weeks or months of careful work. There’s a reason reputable suppliers dominate the references in publications: trust in batch-to-batch consistency and real customer service when things go sideways.
Demand for specialty chemicals like 4-Methyl-5-Formyl Thiazole grows every year. Labs in smaller research institutions and startup environments sometimes struggle to access high-purity chemicals at reasonable cost because they lack purchasing clout. Scientists have faced weeks-long delays, sometimes because of regulatory paperwork, sometimes just supply chain hiccups.
One potential solution comes down to collective purchasing — several labs teaming up to place larger orders and unlock volume discounts. Some institutions foster vendor relationships through consortia that support collective bargaining power, making it easier for smaller research centers to get what they need without overpaying.
I’d also recommend supporting suppliers who invest in prompt customer communication and transparent stock updates on their websites. No researcher likes waiting weeks just to hear if a compound is actually available.
Sourcing high-purity 4-Methyl-5-Formyl Thiazole regularly means requesting certificates of analysis and asking about available purity grades. Researchers I respect verify reported data independently with their in-house analytical facilities to spot inconsistencies before a full batch is ordered. Even reputable vendors can have the occasional mix-up, so double-checking protects your work.
Strong vendor relationships help solve these challenges. I’ve seen institutions lean on their suppliers to expedite shipments, resolve discrepancies, and even arrange custom syntheses if commercial stock runs dry. That kind of support keeps science moving and cuts down on delays that can derail research or innovation.
Choosing legitimate, reputable suppliers for high-purity 4-Methyl-5-Formyl Thiazole isn’t just a bureaucratic formality — it’s a core part of doing responsible science. Open dialogue between vendors and researchers, along with good documentation and an eye for detail, lifts the standard for everybody involved and keeps projects on track.
| Names | |
| Preferred IUPAC name | 5-methyl-2-formyl-1,3-thiazole |
| Other names |
4-Methyl-5-formylthiazole 4-Methylthiazole-5-carbaldehyde 4-Methyl-5-thiazolecarboxaldehyde |
| Pronunciation | /ˈfɔːrˌmɪl θaɪˈəʊzəʊl/ |
| Identifiers | |
| CAS Number | [12408-30-1] |
| Beilstein Reference | 63540 |
| ChEBI | CHEBI:16836 |
| ChEMBL | CHEMBL1914101 |
| ChemSpider | 64806 |
| DrugBank | DB08345 |
| ECHA InfoCard | 08e6cfbe-b643-40b8-9a33-65ae6433dba4 |
| EC Number | 616-907-4 |
| Gmelin Reference | 96913 |
| KEGG | C06171 |
| MeSH | D04103 |
| PubChem CID | 2894134 |
| RTECS number | WN8575000 |
| UNII | 85L54R8X27 |
| UN number | UN3335 |
| Properties | |
| Chemical formula | C5H5NOS |
| Molar mass | 129.16 g/mol |
| Appearance | Light yellow to yellow powder |
| Odor | roasted, nutty |
| Density | 1.21 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 0.67 |
| Vapor pressure | 0.2 mmHg (25 °C) |
| Acidity (pKa) | pKa = 2.46 |
| Basicity (pKb) | 3.63 |
| Magnetic susceptibility (χ) | -66.8·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.563 |
| Dipole moment | 2.81 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 211.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –61.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –2197 kJ/mol |
| Pharmacology | |
| ATC code | '' |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, causes skin irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | > 109°C |
| Autoignition temperature | 180°C |
| Lethal dose or concentration | LD50 oral rat 3200 mg/kg |
| LD50 (median dose) | LD50 (median dose) = 800 mg/kg (Oral, Rat) |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 4-Methyl-5-Formyl Thiazole: Not established |
| Related compounds | |
| Related compounds |
4-Methylthiazole 5-Formylthiazole Thiazole 2-Methylthiazole 4-Methyl-5-(hydroxymethyl)thiazole 4-Methylthiazole-5-carboxylic acid |