Diving into chemical archives, 4-iodoimidazole doesn’t grab headlines like some flashier molecules, but it’s quietly earned respect in synthetic circles. Its story started when researchers tackled iodination of imidazole rings, discovering that sticking an iodine atom onto the four position of the molecule could tune its properties in surprising ways. Early work on halogenated imidazoles in the 20th century paved the way for a methodical approach. As organic chemistry advanced—with new reagents making site-selective iodination less of a gamble—4-iodoimidazole took shape as a reference point for both academic and industrial chemistry.
4-Iodoimidazole, known by a few labels such as 4-iodo-1H-imidazole or imidazole, 4-iodo-, stands out for its simple but effective molecular framework. Chemists pick it for its specific reactivity and the additional heft that the iodine atom brings to the structure. On the market, high-grade samples target those aiming for research or fine chemical synthesis. Purity matters a lot here; even minor contamination influences downstream reactions. Suppliers show up with neat crystalline forms, geared for use not only by chemists but by biologists curious about imidazole analogues.
This compound almost always arrives as a pale, off-white to beige solid. Structurally, it weighs in at a formula of C3H3IN2, with a molar mass close to 209.98 g/mol. Melting starts around 188-191°C, but it's best to watch the specific batch details as gritty impurities shift this a little. Solubility sits in the moderate camp for polar solvents—think DMSO, ethanol, or DMF—while water uptake doesn't impress. Volatility stays low thanks to the iodine’s bulk. Chemically, the electron-withdrawing effect of iodine tweaks the ring’s acidity, opening new doors in coupling reactions as well as cross-coupling based on Suzuki or Sonogashira reactions.
On a reagent bottle, look for specifics like ≥98% purity, lot numbers for traceability, and CAS number 570-92-1. Sourcing from respected chemical vendors, the labeling usually lists hazard information – pictograms warning about potential irritant effects, sometimes with notes on light and temperature storage. Barcode tracking and traceable certificates of analysis become essential for those working in GMP or GLP environments.
Lab routes for preparing 4-iodoimidazole usually follow two main stories. One relies on direct iodination of imidazole, using I2 in the presence of a mild oxidant like hydrogen peroxide or another activating agent. This keeps byproducts to a minimum, but it requires patience and often a few purification steps. The other path uses a Sandmeyer-type reaction, starting with an aminated imidazole, diazotizing and swapping in iodine through copper catalysis. While bulk production scales the first route, custom syntheses often benefit from modifications in solvents, temperatures, and purification tweaks—all depending on what side-products one wants to avoid in the flask.
4-Iodoimidazole doesn’t just sit around waiting for admiration. Its crown feature, the iodine atom, acts as a gateway for diverse C–C and C–N coupling reactions, including well-studied palladium-catalyzed cross-couplings. Chemists find use in the Suzuki-Miyaura and Sonogashira reactions, stitching the imidazole ring onto larger, more elaborate molecules. N-alkylation or substitution at the C5 position follows standard imidazole chemistry, but the presence of the iodine makes selective modification easier to control. In medicinal chemistry, these transformations enable the creation of imidazole-bearing drugs or probes for biochemical assays.
Beyond 4-iodoimidazole, you might spot the names 1H-imidazole, 4-iodo- or simply imidazole, 4-iodo, on catalogs and lab logs. In some literature, abbreviations like 4-IIm pop up, especially in shorthand for cross-coupling screens. Keeping track of synonyms matters for ordering the right compound—no one wants a multi-week delay over a catalog misreading.
Handling 4-iodoimidazole draws on familiar good laboratory practices. Solid dust can irritate skin and eyes; nobody likes a surprise rash after a long day at the bench. Gloves, goggles, and fume hoods count as standard gear. Waste protocols are key—iodinated compounds demand responsible disposal to avoid iodine contamination. Storage at room temperature usually works, but dry conditions lock away hydrolysis worries. Labeling with hazard symbols makes sure newcomers in the lab pay attention before weighing their first batch.
In real-world labs, use cases for 4-iodoimidazole bloom past simple synthetic curiosity. Researchers dig into its potential as a building block for pharmaceuticals, with the imidazole ring showing up in many drugs—antifungals, anticancer agents, enzyme inhibitors. Modifying the molecule using that reactive iodine group lets scientists attach nearly anything: fluorescent tags, affinity probes, or larger organometallic complexes. Analytical chemists have found value in this molecule for labeling reactions or developing standards for high-performance liquid chromatography (HPLC) calibration.
University labs and pharmaceutical companies have kept 4-iodoimidazole under their scopes, searching for the next breakthrough in drug design or catalyst development. Work on optimizing cross-coupling conditions has improved accessibility to otherwise hard-to-make aryl-imidazole hybrids. Structural analog studies compare 4-iodoimidazole with other ring-substituted compounds, mapping how changes affect biological activity. Some R&D projects dive into ways to recycle or recover spent iodinated solvents or wastes, aiming to green up the process chain.
Toxicologists know to respect halogenated organics. Tests on 4-iodoimidazole suggest moderate risk if ingested or absorbed, but acute toxicity looks lower than for some related compounds. Its metabolic pathways in mammals aren’t mapped in detail, so careful record-keeping with dosing and exposure makes a difference in animal studies. Chronic effects haven’t shown up as major worries based on current literature, but every research field has its gaps, especially with niche molecules. Environmental persistence can pose problems; researchers take care that waste ends up in designated collection rather than down the drain.
Looking forward, 4-iodoimidazole seems poised for more attention as new catalysts demand finely tuned building blocks and as drug discovery hunts for diverse ring structures. Advances in photocatalytic or enantioselective transformations might lower cost and improve environmental scores. I expect to see more automated platforms using 4-iodoimidazole as a core scaffold, slicing development time in medicinal chem campaigns. If stricter environmental rules hit halogenated waste, recycling and reuse technologies stand to become a new frontier. For those willing to tinker, 4-iodoimidazole keeps opening doors to more complicated, functional, and useful molecules.
Pulling apart chemistry terms can feel intimidating, but there’s something down-to-earth about tracing the elements that make up a substance. 4-Iodoimidazole offers a good example. Its chemical formula, C3H3IN2, lets us read its atomic recipe like a grocery list: three carbons, three hydrogens, one iodine, and two nitrogens.
A formula like this isn’t random; it shapes everything about the compound’s behavior and how scientists approach it in the lab or industry. By swapping out a single atom—add iodine at the fourth position on the imidazole ring—the whole game changes. Suddenly, a familiar building block in biochemistry looks different, responds to reactions in new ways, and serves unique roles as a chemical intermediate.
Molecular weight grounds a compound in practical reality. For 4-iodoimidazole, the math goes like this:
Add it up, and the molecular weight of 4-iodoimidazole lands at 193.98 g/mol. For anyone moving grams of material around in a flask or scaling up to industrial production, this number means everything. It’s not just trivia—it guides mixing ratios, dosing, and even waste management. Toss 4-iodoimidazole into a reaction, and knowing its mass gives control over outcomes and safety.
Working with chemicals, accuracy matters more than a passing grade from a chemistry teacher. I’ve seen projects stall because someone misread a formula and ordered a related molecule that didn’t work. In pharmaceutical labs, one atom out of place brings legal and safety headaches, not just failed experiments. Commercial producers need the right specifications. Small research outfits run on tight budgets. A mix-up can mean wasted money and time.
Iodine’s presence in 4-iodoimidazole, compared to the original imidazole, draws extra attention. Lab workers suit up and check protocols twice. Safety data sheets flag the risks, especially since halogenated compounds act very differently in biological and environmental settings. The substitutions on an imidazole ring tweak reactivity, metabolic fate, and toxicity.
Errors over chemical names, formulas, and weights still sneak into paperwork and digital files. Technical staff sometimes juggle too many labels or rely on memory, setting up mistakes. There’s a fix in using barcode-based inventory. Software links a unique code to every bottle, tying together chemical name, formula, batch number, and even safety notes. Instead of handwritten notes, everyone scans before using, reducing human error.
Another solution involves more transparent training. Young lab staff pick up habits from experienced colleagues but often miss why every letter and number in a formula matters. Workshops that run through real-world mistakes—wrong chemical ordered, unexpected byproducts, contamination, or health scares—bring the importance home, beyond the textbook.
Safety and efficiency come down to keeping details straight, whether in high-stakes pharmaceutical labs or a university teaching lab. A chemical like 4-iodoimidazole, with its precise molecular identity, calls for respect in every context.
Chemicals rarely forgive sloppy storage—4-Iodoimidazole shows no exception. This compound, known for its role in organic synthesis and pharmaceuticals, thrives on stable conditions. Years spent working in university labs have drilled one lesson home: even small missteps get expensive and risky fast. Too much humidity or the wrong jar turns useful chemicals into trash—4-Iodoimidazole likes to remind chemists of this rule.
Exposure to air brings out a cruel side in many organic compounds. 4-Iodoimidazole, with its heavy iodine atom, faces quicker breakdown than its non-halogen cousins. Air and moisture team up to start slow, silent reactions. Yellowing powder, odd odors—these are giveaways that something’s gone wrong. Light brings a different pain. Direct sunlight, or even strong indoor bulbs, can nudge iodine around or spark decomposition.
Simple glassware on an open shelf just invites trouble. In a pinch, I’ve seen people wrap vials with aluminum foil and stash them in the fridge—not elegant, but better than letting an expensive flask of fresh material lose value. 4-Iodoimidazole prefers the standard cool, dark spot many sensitive chemicals demand. This isn’t about obsessing—it’s about keeping dollars and data safe.
Open the cap too often and it’s easy to overlook what’s drifting inside. Troops of water molecules in the air will latch on fast, turning a dry powder into a clumpy, ruined mess. I’ve dealt with the fallout of old containers with swollen, useless contents. Keeping 4-Iodoimidazole dry cuts out unnecessary stress. Drierite or silica gel packs in the same container help. In crowded labs, a desiccator cabinet or a sealed bag does as much good as fancy equipment. Simple habits—seal it tight, open only in dry air—stretch the life of every gram.
Truth is, shelf lives for chemicals like 4-Iodoimidazole often go untested in real-world labs. Official vendors talk about 2 to 3 years under proper conditions, but I’ve seen lots survive longer. The catch: every time that cap turns, every day a fridge sits in defrost mode, that lifespan shrinks. Frequent temperature swings bring moisture into play. Watch for signs—color change, strange smells, or caking—and don’t trust old material with important experiments.
Stocking too much for fear of running out hurts more than it helps. Smaller orders, matched to reasonable project timelines, avoid waste and shelf decay. Sharing supplies within research groups keeps things moving before chemicals get old. If using 4-Iodoimidazole for regulated work or production, keep tight batch-tracking and refresh supplies early. Automated temperature and humidity logging pays off with high-value reagents. Lab experience keeps pointing in the same direction—simplicity, care, and attention stretch budgets and results further than any miracle material.
If you step into a synthetic organic chemistry lab, there’s a good chance you’ll spot someone pulling a vial of 4-Iodoimidazole off the shelf. For folks deep into molecule-building, this compound stands out for its versatility. Its base structure comes from imidazole, a molecule chemists love for building bigger, useful things, and that core gets a twist when iodine enters the picture. This small swap changes the way scientists think about what they can do with it. I’ve seen a few seasoned researchers in pharmaceutical development stash it among their “essentials” because it opens so many doors during drug synthesis.
Let’s get concrete about drug discovery. Every time a team needs to tack on a complex group to the imidazole ring, 4-Iodoimidazole enters the mix. That iodine atom turns the whole molecule into a perfect participant for Suzuki-Miyaura coupling—a reaction at the heart of how many new chemical backbones are put together. It acts like a “handle”: chemists can practically snap on different groups and watch new compounds emerge that just might become next-generation medicine. In my experience reading journals and reviewing synthesis protocols, researchers trying to broaden the library of antivirals, antifungals, or even some experimental antipsychotic drugs have all leaned on this very compound.
It’s not only new medicines that make 4-Iodoimidazole valuable. In biotechnology, this molecule serves as a base for building probes and labels. Scientists want to watch what’s happening inside a living cell, so they add fluorescent groups to molecules like imidazole—thanks to that handy iodine, the process gets smoother and more reliable. I’ve heard folks working in protein tagging speak about how much simpler life gets when their starting materials cooperate through straightforward coupling reactions. Students running enzyme studies and imaging experiments get dependable results, and the lab schedule doesn’t explode.
Current talks around smart sensors or next-level electronics often feature molecules that sound obscure, but 4-Iodoimidazole turns out to be surprisingly helpful. People in materials science adapt it for crafting organic semiconductors or thin films. From forum discussions and a couple of hands-on workshops, it’s clear that the iodine group assists with laying down molecules in specific patterns. That means higher-performance organic circuits or even simple chemical sensors can take shape more easily. Startups testing flexible electronics usually have at least one story about using molecular building blocks like this.
Lab life gets stressful without reliable building blocks, especially in projects focusing on chemical detection for the environment. Analytical chemists searching for traces of pollution rely on special reagents for their detectors, and customized imidazole derivatives step in for the job. 4-Iodoimidazole lets them quickly create unique marker compounds that highlight even low concentrations of hazardous substances. A few reports coming out of university labs in urban areas have charted how researchers use this approach to screen for heavy metals or strange industrial byproducts.
No one likes bottlenecks, especially when new treatments or technologies hang in the balance. One thing I keep noticing is that easier access to high-quality 4-Iodoimidazole streamlines the process for experts in different fields. If suppliers lower costs through better manufacturing or offer greener synthesis routes, more research teams can test creative ideas—whether they chase a new diagnostic technique or ramp up the push for smarter materials. Sometimes, the solution for bigger challenges starts with one reliable chemical in the hands of motivated people.
You take a scoop of 4-iodoimidazole, dump it into some water, give it a swirl. Not much happens. The stuff lingers, settles to the bottom, barely gives you a hint of dissolution. For chemists at the bench, that means wasted time fighting clumps that refuse to budge, instead of getting ready for the next step in a synthesis. Water isn’t going to save you here. Most researchers I’ve talked to just sigh and reach for something else. What makes this especially important is that a lot of biological and pharmaceutical work leans on water-based systems. If your compound doesn’t play nice with water, you start behind the line.
We all want one easy answer: just switch the solvent and carry on. In practice, things rarely go that smoothly. Classic go-tos like ethanol or methanol don’t break the stalemate. Acetone? The solid still laughs in your face. Solvents with similar polarity rarely fare better. Step into the more exotic, and you risk introducing new problems—cost jumps up, safety protocols balloon, and environmentally speaking, things get dicey. With 4-iodoimidazole, the range of solvents that truly dissolve it shrinks, and the ones that work often fit the “special order” bill only big labs can afford.
People outside research circles might roll their eyes at chemists griping about solubility, but for folks developing new medicines or imaging agents, every insoluble lump can bring a project to a halt. I’ve watched researchers spend hours trying every trick in the book: heating, sonication, back-of-the-napkin ideas like adding a pinch of salt or a splash of acid. Weak solubility leads to bad yields, wasted materials, and frustration. We aren’t just talking about inconvenience—solubility issues can push labs to abandon promising molecules entirely.
It boils down to the balance between its imidazole core and that bulky iodine sticking out. Normally, imidazoles play pretty well in water; biochemistry textbooks are littered with examples. Stick that hefty iodine on the ring and suddenly the harmony crashes. The molecule becomes more polarizable and less inclined to cozy up to solvent molecules. That’s not just theory—I’ve seen students stare at undissolved 4-iodoimidazole for hours, only to sheepishly scrape unused powder out and move to plan B.
If you’re stubborn enough to keep going, there are a couple things that might help. Some colleagues dissolve it in dimethyl sulfoxide (DMSO) or N,N-dimethylformamide (DMF), though both come with their own headaches—cost, disposal, of course the ever-present stink of DMSO. Using co-solvents sometimes offers a shortcut, combining a splash of DMSO and water to coax the 4-iodoimidazole into solution. For scale-up on pilot or industrial levels, grinding the compound finer can help, and I’ve watched folks warm the mixture just enough to nudge things along, though one always has to worry about heat-sensitive side reactions.
The solubility battle isn’t just a lab annoyance. It shapes which molecules get used in clinics, which discoveries hit roadblocks, and how much waste piles up at the end of the week. If chemists could coax more solubility from stubborn compounds like 4-iodoimidazole, or design alternatives with the same properties minus the hassle, the pace and accessibility of drug development could pick up. Less time fighting insoluble gunk means more time chasing real breakthroughs.
4-Iodoimidazole sounds like a complex lab compound, and in many ways, it is. This chemical has made its way into research circles, especially where organic synthesis matters, such as medicinal chemistry. Yet, its chemical structure — with an iodine atom tucked into the imidazole ring — brings up a few concerns for anyone pouring it out of a glass bottle. Handling these powders or crystals can't be treated the same as salt in the kitchen.
Anyone who's spent time in a wet lab knows not all reagents are created equal. No one writes songs about 4-iodoimidazole, but it does belong with a group of substances that can cause issues on contact or inhalation. I’ve seen researchers develop skin irritation or get a nasty cough from getting careless around similar compounds. The Material Safety Data Sheet for this substance flags it for causing skin and eye irritation. Breathing in any sort of powder — especially one containing iodine and aromatic rings — isn’t smart.
In a rush, it’s tempting to grab a pipette with bare hands, especially if the job takes just a minute. That habit leads to regrets. 4-Iodoimidazole should always be handled with gloves—nitrile does the trick here. Splashing it in your eyes or letting it touch your skin brings enough risk of irritation that good goggles matter. Labs call for not just a lab coat, but closed-toe shoes, a splash shield if you’re clumsy, and tied-back hair. Sounds basic, but skipping any one of those makes accidents more likely.
Many chemicals, even those that don’t stink up the place, should only be measured out in a fume hood. From experience, working outside of one is just asking for a surprise. Aromatic imidazoles can give off vapors when handled in bulk or under heat. That’s not what anyone wants near their face. Fume hoods lower the chance of breathing in particles and remind everyone else in the room to stay sharp. Working in open air just isn’t worth it.
Some of the worst day-to-day lab messes come from careless storage. Putting 4-iodoimidazole in a loosely capped bottle or under bright light sets up problems. This compound, like many similar substances, stays happiest and safest in a tightly sealed amber glass bottle, tucked away from direct sunlight and strong bases or acids. If a container cracks or a spill happens, the proper way to clean up involves damp paper towels (to minimize dust) and immediate disposal in a labeled chemical waste bag.
Having been the safety officer in a crowded student research lab, I saw how experienced chemists can get complacent. Making safety routines second nature really cuts down on accidents. Regular reminders about labeling, logbook entries, and not mixing up spatulas go a long way. Encouraging others to speak up about small incidents creates a safer space for everyone, whether they’re handling 4-iodoimidazole or some other niche reagent.
The basics work for a reason. Gloves, googles, and a working fume hood. Training students and new staff on the right way to open, weigh, and dilute strange powders makes life easier for everyone. Clear signage, up-to-date safety sheets on the wall, and a habit of logging every gram used helps trace problems quickly. Investing in spill kits and fire extinguishers keeps labs safer, even if no one expects to need them.
Every new or rarely used chemical on a lab shelf deserves respect. 4-Iodoimidazole shouldn’t be the scariest thing in the room, but treating it lightly never ends well for long. Building simple, direct safety habits pays off every single day.
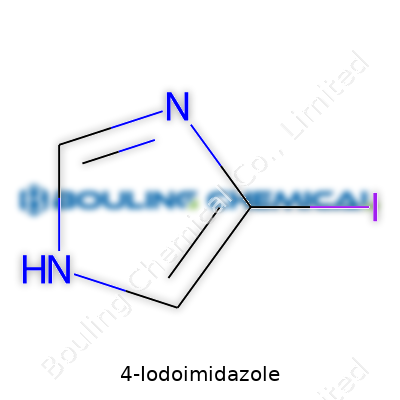
| Names | |
| Preferred IUPAC name | 4-iodo-1H-imidazole |
| Other names |
1H-Imidazole, 4-iodo- 4-Iodo-1H-imidazole 4-Iodoimidazole |
| Pronunciation | /ˈfɔːr-aɪˈoʊdoʊ-ɪˈmɪdəˌzoʊl/ |
| Identifiers | |
| CAS Number | 3430-80-0 |
| Beilstein Reference | 120803 |
| ChEBI | CHEBI:38482 |
| ChEMBL | CHEMBL228029 |
| ChemSpider | 115798 |
| DrugBank | DB08302 |
| ECHA InfoCard | ECHA InfoCard: 100.040.188 |
| EC Number | 1.3.1.99 |
| Gmelin Reference | 83795 |
| KEGG | C08667 |
| MeSH | D016692 |
| PubChem CID | 69704 |
| RTECS number | NL1575000 |
| UNII | 4U2I1313FB |
| UN number | UN3276 |
| CompTox Dashboard (EPA) | 4-Iodoimidazole |
| Properties | |
| Chemical formula | C3H3IN2 |
| Molar mass | 208.01 g/mol |
| Appearance | White to light yellow crystalline powder |
| Odor | Odorless |
| Density | D=2.46 |
| Solubility in water | Soluble in water |
| log P | 0.2 |
| Vapor pressure | 0.0000455 mmHg at 25°C |
| Acidity (pKa) | 5.39 |
| Basicity (pKb) | 11.74 |
| Magnetic susceptibility (χ) | NA |
| Refractive index (nD) | 1.712 |
| Dipole moment | 2.96 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 132.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -11.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -185 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | V09AX03 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07, GHS09 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | H315, H319, H335 |
| NFPA 704 (fire diamond) | 3-1-0 |
| Flash point | Flash point: 142.3°C |
| Lethal dose or concentration | LD₅₀ (oral, rat) > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1470 mg/kg (rat, oral) |
| NIOSH | Not established |
| PEL (Permissible) | Not established |
| REL (Recommended) | 100 ppm |
| Related compounds | |
| Related compounds |
Imidazole 2-Iodoimidazole 4-Bromoimidazole 4-Chloroimidazole 4-Methylimidazole |