Long before most people thought about what goes into pharmaceuticals or specialty polymers, chemists in the early-to-mid 20th century were tinkering in labs and stumbled upon a compound called 4-hydroxypiperidine. Back then, the field was buzzing with advances in organic synthesis, and the piperidine ring was gaining some serious attention. Folks noticed that substituting a hydroxyl group on the fourth carbon did remarkable things to the molecule’s behavior. Laboratories ran with this, experimenting with routes built off older piperidine syntheses. For years, that motivation came from drug discovery programs and materials segments chasing after new properties and performance. The foundation these scientists laid still shapes how people manufacture and use 4-hydroxypiperidine today.
Ask around in chemical supply circles, and you’ll hear 4-hydroxypiperidine described as a colorless to pale yellow liquid or crystalline solid, depending on the grade and temperature. Its structure—a six-membered ring with a nitrogen and a hydroxyl group—shows just enough flexibility and reactivity for it to play well in highly specialized settings. You find it in bulk drums for industrial users or in sealed vials for research. What stands out is its popularity as a building block; drug makers, materials chemists, and academic labs snap it up because it plugs easily into synthesis pathways without too many headaches.
This is not just another ring-shaped molecule with a quirky name. 4-hydroxypiperidine has a melting point hovering around 32-35°C and boils out in the 210-215°C range, which gives process engineers some breathing room for different routes and storage options. Its solubility in water and organic solvents opens doors for both aqueous and nonaqueous chemistry. The hydroxyl group at position four sticks out enough to make the molecule both a hydrogen bond donor and acceptor, which turns out to be crucial in designing derivatives and assembling more complex molecular frameworks. If you spill some on your hands, you’ll immediately notice a slippery texture—a subtle reminder of the molecule’s basicity and the potential hazards involved.
Every drum or bottle of 4-hydroxypiperidine comes labeled with details: CAS number 626-56-2, purity levels reaching or exceeding 98% for most research purposes, and hazard icons warning about toxicity or risks for skin and eye irritation. The UN and DOT numbers on the side tell you about transport restrictions. Manufacturers list pH range, assay methods, and storage recommendations straight on the data sheet. Temperature-sensitive batches demand clear ‘keep cool’ instructions so that buyers know to stash them in climate-controlled spaces, avoiding sunlight and moisture exposure.
Most commercial processes for making 4-hydroxypiperidine start with piperidine itself or protected piperidone intermediates. A favorite lab route runs through catalytic hydrogenation followed by selective hydroxylation, usually using well-known oxidation reagents like potassium permanganate or chromium trioxide. Some routes dodge the chromium and lean on more sustainable oxidants, which makes a difference for those of us working on greener practices. Scaling from grams to kilograms shifts focus to yield, cost, and cleanliness, so manufacturers tweak each step, trading between route efficiency and purification complexity.
4-hydroxypiperidine’s chemistry centers around its dual functionality. The nitrogen atom in the ring acts as a nucleophile, and the alcohol group at position four opens a world of substitution and condensation reactions. You see this compound alkylated or acylated in the lab, feeding straight into the production of advanced pharmaceutical ingredients, specialty surfactants, or as part of chiral auxiliary systems. One common move links the piperidine to aromatic rings, building motifs that pop up in painkillers or anti-psychotic drugs. Oxidation, reduction, and cyclization are all fair game for inventive researchers looking to tweak biological activity or physical properties.
Chemists love their aliases, and 4-hydroxypiperidine is no exception. Walk through a catalog and you’ll spot names like Piperidin-4-ol, 4-Piperidinol, and even 4-hydroxy-hexahydropyridine. Each name might target a different audience, but the substance inside is the same backbone. Suppliers sometimes list it by its registry number or short-hand abbreviations, which adds a bit to the confusion for new players in the industry. For shipment and customs, names like UNII-8QGIK6LXHF or EC No 210-951-8 show up, helping trace the compound through regulatory systems worldwide.
A healthy respect for 4-hydroxypiperidine pays off in any laboratory or factory. Even modest exposure can irritate skin and eyes, so gloves and goggles are automatic. Inhalation ticks up health risks—fume hoods or local exhaust blowers work best for handling open volumes. Spills on benches clean up with absorbent pads and lots of water, but solvents must never head down public drains. Safe storage keeps temperatures stable and containers tightly capped, often with a desiccant nearby to tackle moisture. Shipping includes the right hazard codes and packaging, tracking the contents and exposure potential right through to the end user.
Pick up a modern drug patent or polymer formulation sheet, and the chances are high you’ll spot 4-hydroxypiperidine on the ingredient list. It serves as a precursor for a host of pharmaceuticals, including antipsychotics, analgesics, and antitussives. In the lab, it stands as a favorite scaffold for target-oriented synthesis of complex molecules. Industrial coatings, personal care products, and even agrochemicals occasionally borrow this compound for its chemical adjustability and stability. During my graduate years, I watched colleagues rotate its derivatives into combinatorial chemistry screens looking for new biological leads—a testament to its adaptability across scientific fields.
R&D teams keep coming back to 4-hydroxypiperidine because it bridges classic synthetic methods with modern innovation. Medicinal chemists fine-tune analogues to shift solubility or cell permeability; materials scientists stretch the boundaries by building it into new block copolymers. Analysts probe how subtle tweaks around the ring trigger different pharmacological activities. Newer methods in green chemistry emphasize less waste and cleaner workups; institutes roll out patented processes that use milder oxidizers or biocatalysis. Progress here directly feeds into downstream sectors, giving companies an edge in crowded markets.
Toxicologists pay strict attention to 4-hydroxypiperidine’s performance in vivo and in vitro. Animal studies flag neurotoxicity at high doses; researchers note central nervous system effects—headaches, dizziness, and, in larger quantities, seizures. Chronic exposure risks remain under active study, especially in environments with poor ventilation or lax personal protective measures. Laboratory assessments monitor both acute and sub-chronic dosing levels, looking for warning signs that feed into regulatory guidelines. Waste disposal and accidental release protocols prioritize containment, neutralization, and controlled incineration to limit broader ecological impact.
Growth for 4-hydroxypiperidine doesn’t slow down. Upstream supply chains in Asia and Europe continue to expand, and innovators keep hunting for variants that outperform their predecessors in therapeutic or industrial roles. The push toward more sustainable and less hazardous production lines motivates continuous improvement, while digital modeling and AI-assisted drug design spotlight new derivatives faster than ever. As new therapeutic targets and advanced materials emerge, this versatile molecule’s importance will only become more apparent. Both startups and established players pour resources into patent filings, pre-clinical research, and scalable production, nudging 4-hydroxypiperidine’s relevance well into the next generation of science.
Step into any research lab focused on pharmaceuticals, and you’ll probably find a small jar marked "4-hydroxypiperidine" on some shelf. This isn’t some rare chemical that only big drug makers deal with. It shows up in plenty of research places that develop new medicines, especially for mental health and pain relief. If you’ve ever read the label on a prescription drug for depression or certain nerve disorders, there’s always a chance its core structure owes something to 4-hydroxypiperidine.
People in drug development lean towards chemicals that are easy to modify and give predictable results. 4-Hydroxypiperidine has that drawing power. Its chemical structure opens up opportunities for building complex molecules, which leads to new medicines. Drug companies take advantage of this, using it as a building block to create treatments for depression, Alzheimer’s, or even schizophrenia.
I’ve sat in discussions where researchers sorted through which parts of a molecule worked and which didn’t. There’s always the same story: finding a piece that connects to a target in the body is half the battle. 4-Hydroxypiperidine connects easily. Some new antipsychotic drugs rely on variations of this compound because chemists can easily attach new side chains and tweak its properties, making drugs that stick to serotonin and dopamine receptors better.
It’s not just drug makers turning this compound into something useful. Agrochemical developers have started looking at it, too. Pesticides and herbicides get their kick from molecules that disrupt certain functions in bugs or plants. Here, 4-hydroxypiperidine serves as a launching pad to make those molecules. That helps boost crop yields and manage pests with less chemical waste.
One concern jumps out. Widespread use of 4-hydroxypiperidine means there’s a steady flow of it through labs and some chemical supply houses. In the wrong hands, any compound that helps build complex molecules can get misused. Regulation and tracking have to stay tight. It’s not paranoia—history taught us the hard way that some drug precursors wound up sidestepping the rules, leading to dangerous outcomes.
Changing how this compound gets managed could ease problems. Designating it as a watched substance would make supply chains more accountable. At the same time, the chemistry community has a habit of sharing information, even if it’s about the risks. Anyone working in the lab should keep safety and traceability up front. Responsible handling and open conversations among scientists help keep accidents and misuse in check.
Still, the benefits are hard to ignore. Every year, new medicines based on 4-hydroxypiperidine hit the market. Seeing friends and family get support for depression or chronic pain reminds me why keeping this work moving is so vital. We need policies that balance innovation with safety, so new therapies keep coming without leaving communities exposed.
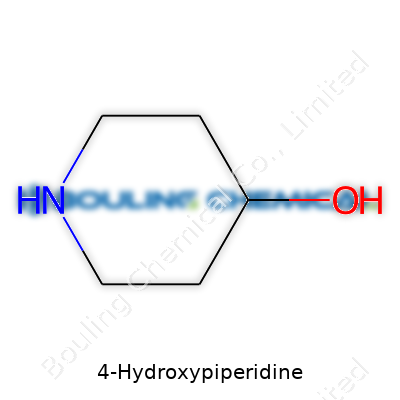
4-Hydroxypiperidine shows up now and then when talking about pharmaceuticals or synthetic chemistry. The name might sound intimidating if you’re not a chemist, but its backbone stays straightforward. Picture a six-membered ring made of five carbon atoms and one nitrogen. That’s called piperidine. Now add a single –OH group (a classic alcohol group) right on the fourth carbon. Together, you get 4-Hydroxypiperidine.
Looking at chemical shorthand, the piperidine ring takes on the formula C5H11N. With a hydroxyl (–OH) attached at the fourth slot in the ring, the total switches to C5H11NO. Chemists often draw it with the nitrogen atom off at one corner of the ring, with numbering starting next to the nitrogen and moving clock-wise until they hit the fourth carbon. Drop the alcohol group there, and that’s your basic chemical structure. For those who turn to lines and bonds, piperidine remains a common sight, and the –OH group stands out on the drawn ring as a little offshoot.
This molecule ends up pretty valuable. Its ring and that hydroxy group leave just enough chemical “hooks” for other groups to attach and build useful products. Drug designers pick 4-hydroxypiperidine when crafting therapies targeting neurological issues or pain pathways. They rely on the ring to mimic amino acids or neurotransmitters in the body, and 4-hydroxypiperidine can act like a shape-shifting building block. In my university lab, anyone who worked with this compound always wore gloves: the basic ring can irritate skin, and the nitrogen atom has a knack for attracting other molecules, making it reactive in the right chemical soup.
It’s not just biology. Industrial chemistry steps in, too. Piperidine derivatives help with producing some polymers, specialty adhesives, and even corrosion inhibitors. 4-Hydroxypiperidine sits near the source of those specialty chemicals since that hydroxy group means you can hang even more functional pieces on the ring. Think of it as a Lego piece with an extra peg sticking out.
Building 4-hydroxypiperidine takes some patience. Off-the-shelf versions don’t always offer the purity or price that works for scale. Most labs start with piperidine itself. The trick involves selective oxidation at the fourth spot, which sounds simple but takes careful control. Chemists have to avoid over-oxidation; otherwise, they end up busting the ring entirely or attaching groups in the wrong spots. Some processes use protective groups or catalysts, but that means more steps and higher costs. I remember a friend complaining about the smell of piperidine in the air when running these reactions—turns out, the stuff carries a strong, fishy funk no matter how well the fume hood works.
Labs worldwide keep searching for smarter ways to attach that hydroxy group. Cleaner, one-step methods cut down on energy waste and create less chemical trash. Companies already use greener solvents and enzyme-driven methods, and these new methods promise fewer headaches and lower bills. Funding in green chemistry sparked hopes for using renewable feedstocks and “bio-inspired” processes, but established industrial routes just don’t switch overnight.
If you care about molecule design, human health, or even pollution, the structure of 4-hydroxypiperidine stands out for its impact and its challenges. No matter the use, getting the formula right starts with understanding—and making—the ring itself.
Any chemist who’s cracked open a new container of 4-Hydroxypiperidine will recognize the subtle whiff of something potent drifting from the bottle. Folks in the business know: this isn’t the sort of material you toss on any old shelf and forget about. Stories float around labs about what happens when things aren’t put away right—painted steel shelves corroding from a simple spill or rubber stoppers cracking from vapors. Even if you’re not running a kiloton operation, good storage keeps a workspace calm and avoids trouble down the road.
Anyone who has worked a late-night shift in a university lab knows how much havoc a leaky cap or a warm corner can create. 4-Hydroxypiperidine, with its strong tendency to draw water from the air and react with oxygen over time, amplifies these hassles. Left in a steamy storeroom or in sunlight, the compound loses its punch and causes headaches for the next researcher looking for consistency. Every ruined sample is another late night repeating a failed experiment—which isn’t just a waste of effort; it’s a waste of money and resources.
Reliable storage isn’t about fancy chillers or high-tech solutions. The biggest gains come from closing the lid properly, picking the right spot, and keeping things organized. This compound likes cool, dry spots—think 2-8°C, common for most lab refrigerators. Shelving away from heat pipes, exhaust fans, or windows makes a huge difference. It’s surprising how quickly sunlight or warmth sneaks in, especially in crowded basements or makeshift stockrooms.
Over the years, I’ve worked with lab teams who tried everything: desiccators stuffed with fresh drying agents, zip-locked containers inside airtight cabinets, and yes, even double-bagging with thick freezer bags to keep the moisture out. These steps seem basic, but even one careless hour out on the bench ruins a bottle’s contents. The jokes about “lab gremlins” exist for a reason, but storage mistakes nearly always trace back to simple human error.
4-Hydroxypiperidine gets along poorly with many common lab items—even humble cardboard can turn mushy after long exposure. Polyethylene or glass bottles with tight-fitting caps outlast others. Wherever possible, small aliquots keep the main supply from repeated openings. Nobody wants to find a greasy, leaking bottle at the back of a forgotten drawer months later.
Spilled or degraded chemical doesn’t just mean a bad day for the researcher. Those responsible for workplace safety will confirm that fumes can trigger lab evacuations or worse. Some compounds evaporate more quickly than expected, especially under the heat of summer, turning an easy job into an emergency cleanup.
Facility managers insist on detailed labeling and inventory checks for a reason. A clear date on every bottle cuts down on mystery liquids and expired stocks lurking in the wrong spot. Colleagues who take two minutes to jot down where and how they stored a batch save everyone headaches in the long run. A sturdy, labeled fridge stands as the best investment for anyone handling 4-Hydroxypiperidine—even if just for peace of mind.
None of this takes special training or costly equipment. Good habits, basic containers, and a little awareness. That’s been the difference between the smooth flow of a well-managed lab and the chaos of last-minute spill response. Simple fixes make lots of wearisome problems vanish before they ever start.
4-Hydroxypiperidine isn’t a household name. I’ve come across it mostly in lab settings, where folks handle it for pharmaceutical development and chemical research. The first thing you learn around any chemical is respect. Not alarm, but respect. This compound falls under that common sense rule pretty quickly. You open up a safety data sheet and see words like “harmful if swallowed” or “causes skin irritation.” That’s not marketing hype; it’s the kind of warning you only ignore if you’re itching for trouble.
Contact with 4-Hydroxypiperidine won’t land you in the hospital on the spot, but spill it on your skin and you’ll know you’ve messed up. Most datasheets back up this stingy reality. Redness on skin, irritation in the eyes, maybe a sore throat after breathing in too much of the stuff. These issues are rarely severe when handled right, but ignoring gloves, skip goggles, or sniff around the bottle and you pick up what it means to get careless.
Plenty of lab veterans remember their first “reminder” about chemical safety. That reminder often stinks, stings, or both. My memory is of a tiny splash during cleanup, forgetting to double-check the gloves. Hours itching and a sheepish trip to the campus clinic. That’s pretty mild compared to stories from folks who take bigger risks.
Digging into toxicology data, you won’t find giant piles of studies about 4-Hydroxypiperidine in the water supply or causing mysterious cancer clusters. It’s not common out in everyday life. What does exist points more to acute hazards: short-term issues right after exposure. Some animal studies suggest toxicity with large amounts, but the regular lab experience puts the main risks at skin, eye, and respiratory irritation.
Some people wonder if not much data equals “safe.” Working with chemicals long enough taught me the opposite. Just because a substance isn’t famous for disasters doesn’t make it harmless. Calling something “of low toxicity” often just means no one made a big headline with it yet. Precaution wins every time, especially with something you can’t buy at the corner store.
The hardest part isn’t the substance itself; it’s the attitude around it. Cutting corners in labs, rushing to finish, or skipping basic training—those decisions guarantee trouble more than the bottle in the cupboard. Accidents come up most where procedures slip. It has less to do with chemistry and more to do with culture.
I’ve worked in places where people treat the safety binder as bathroom reading. The difference shows up in how spills get handled, how well folks remember what to do in a pinch, and what happens when a new student asks questions. One solution starts with solid, no-nonsense safety education. Not just the “read and sign” stuff, but hands-on, scenario-driven drills. Reinforcing good storage habits and regular equipment checks goes a long way. Management has to set the tone—if a boss shrugs at safety, so will the team.
There’s no magic fix for risky chemicals, but that’s no excuse for treating them lightly. Sharper awareness, better habits, open talk about risks—those matter more than memorizing one more data sheet. Honest work keeps 4-Hydroxypiperidine just another tool on the shelf, not a headline.
4-Hydroxypiperidine might sound like one of those chemicals you’d only find in obscure journals, but its fingerprints are all over pharmaceutical labs and specialty chemical plants. It’s not just about the structure—a six-membered ring with a punchy hydroxyl group—scientists and manufacturers treat this compound as a toolbox. Some chemists talk about molecules as if they are just puzzle pieces, but 4-Hydroxypiperidine actually helps build bigger, more interesting molecules. It plays a starring role in many reactions that whip up medicines, fine chemicals, and research probes.
If you dig through patents and syntheses for new drugs, 4-Hydroxypiperidine pops up in more than just a handful. Take antihistamines, antidepressants, and some HIV medications; researchers use this compound for constructing complex rings and backbones that give these drugs their power. I remember talking to a chemist who worked on a new antipsychotic, and he told me the project would have fallen flat without stable intermediates based on 4-Hydroxypiperidine. It’s the go-to scaffold for making small tweaks to molecular shapes, which can change how a medicine interacts with the body. The pharmaceutical industry doesn’t just value the compound because of its chemistry—its availability and affordable price let teams test new ideas quickly.
Beyond drug creation, specialty chemical companies grab 4-Hydroxypiperidine for the job of making advanced polymers and coatings. The compound introduces rigidity, flexibility, or functionality, depending on what’s bolted to the molecule. For example, it’s a core building block in certain additives that protect plastics from breaking down under sunlight. Manufacturers of agricultural chemicals also turn to this ring system to fine-tune pesticides, fungicides, and other crop-aiding products. In flavors and fragrances, it acts as a stepping stone for producing complex aroma molecules that would otherwise be difficult to build efficiently.
Organic chemists look for starting points that allow for creativity and efficiency. 4-Hydroxypiperidine fits this bill. It offers both the nitrogen and the oxygen, which means it can link up with many other building blocks. It’s especially useful for making constrained molecules with fascinating biological activity. In medicinal chemistry, this opens the door to tailor-make drug candidates and study what works and what doesn’t, faster than ever. Over the years, more journals and companies have documented new uses: swapping out the hydroxyl for other functional groups, using the nitrogen as a handle for further modifications, and exploring its role in metal complexes. If you spend time on a busy organic chemistry bench, you know there’s always a bottle of piperidine derivatives close at hand.
Handling 4-Hydroxypiperidine poses practical challenges. It can be volatile, and without proper storage, it picks up water or reacts with air. Chemical spills don’t wait for the end of a shift—so stricter safety protocols and advanced ventilation come into play, especially in larger operations. Scaling up from grams in the lab to kilos on the plant floor tests both the supplier’s reliability and the team’s process design. More sustainable synthesis routes, such as using greener solvents or skipping unnecessary protective groups, are gaining support. For research, sharing protocols and troubleshooting guides online cuts down on wasted resources and keeps progress moving. Chemists who pass along tips for working with tricky intermediates make life easier for the next team chasing the next big compound.
| Names | |
| Preferred IUPAC name | Piperidin-4-ol |
| Other names |
Piperidin-4-ol 4-Piperidinol 4-Hydroxy-piperidine 4-Hydroxypiperidine Piperidine-4-ol |
| Pronunciation | /fɔːr-haɪˈdrɒksi-pɪˈpɛrɪdiːn/ |
| Identifiers | |
| CAS Number | 123-87-7 |
| Beilstein Reference | 115925 |
| ChEBI | CHEBI:33164 |
| ChEMBL | CHEMBL18752 |
| ChemSpider | 61947 |
| DrugBank | DB08320 |
| ECHA InfoCard | ECHA InfoCard: 100.007.318 |
| EC Number | 200-190-6 |
| Gmelin Reference | 7977 |
| KEGG | C02261 |
| MeSH | D010871 |
| PubChem CID | 10427 |
| RTECS number | TC6655000 |
| UNII | M8ZXJ3EK5G |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C5H11NO |
| Molar mass | 87.141 g/mol |
| Appearance | Colorless to slightly yellow liquid |
| Odor | Amine-like |
| Density | 0.997 g/mL at 25 °C (lit.) |
| Solubility in water | Miscible |
| log P | 0.09 |
| Vapor pressure | 0.34 mmHg (25°C) |
| Acidity (pKa) | 10.8 |
| Basicity (pKb) | 4.70 |
| Magnetic susceptibility (χ) | -58.4·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.484 |
| Viscosity | 7.4 cP (20°C) |
| Dipole moment | 2.51 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 150.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -159.9 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4276.3 kJ/mol |
| Pharmacology | |
| ATC code | N04BX13 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye damage, causes skin irritation |
| GHS labelling | GHS02,GHS05,GHS07 |
| Pictograms | ⟦⟬N⟭1CCOCC1⟧ |
| Signal word | Warning |
| Hazard statements | H314: Causes severe skin burns and eye damage. |
| Precautionary statements | P264, P270, P273, P280, P301+P312, P305+P351+P338, P330, P501 |
| NFPA 704 (fire diamond) | 3-3-2 |
| Flash point | 101 °C |
| Autoignition temperature | 310°C |
| Lethal dose or concentration | LD50 (Oral, rat): 528 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 475 mg/kg |
| NIOSH | TE1996000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 14 to 30°C |
| IDLH (Immediate danger) | No IDLH established. |
| Related compounds | |
| Related compounds |
Piperidine Piperidone 4-Piperidone Piperonal |