Scientific curiosity and the drive to create better chemical building blocks brought 4-Hydroxy Piperidine into focus back in the 1960s, when researchers began examining piperidine derivatives for their promise in pharmaceuticals and organic synthesis. Chemists quickly realized that swapping out certain atoms in the piperidine ring could dramatically shift reactivity, which led to the hydroxyl group being added at the four position. Early records find this compound turning up in journals as scientists explored ways to tweak drugs for better activity or reduce side effects. Labs stuck with it because it offers both a handle for further modification and strong stability, unlike trickier groups that fall apart under reaction conditions. The journey from an academic curiosity to a staple in med-chem toolkits wasn’t overnight, but 4-Hydroxy Piperidine benefitted from persistent work by teams who wanted more robust, predictable scaffolding for experimental drugs. More recently, patents for new therapies or catalyst systems feature this compound, testament to its long-standing role in applied science.
4-Hydroxy Piperidine stands out as a six-membered, nitrogen-containing heterocycle, with a single hydroxyl group attached to the fourth carbon. In my own experience, working with synthetic intermediates, this molecule proved useful as an anchor for expanding chemical diversity. Both research and manufacturing settings regularly use solid forms or concentrated solutions, generally supplied by chemical vendors clear on purity, moisture content, and melting point. Laboratories choose it for the reliability—batch-to-batch consistency gives chemists what they need for reproducible results, especially when building up more complex targets like CNS-active agents.
At room temperature, 4-Hydroxy Piperidine shows up as a white crystalline solid, with a melting range typically noted around 41-43°C. It dissolves well in water, moderate alcohols, and many polar solvents, which makes it handy for multi-step synthesis. Structurally, the piperidine ring brings basicity thanks to the nitrogen atom; the hydroxyl group at C4 raises polarity and hydrogen-bonding strength. Chemical stability even under mildly acidic or basic conditions means storage doesn’t require extraordinary steps. In practical terms, that sort of robustness reduces downtime in the lab. The free amine offers sites for N-alkylation, acylation, or salt formation, while the alcohol group can get converted to esters or oxidized, bringing versatility in downstream chemistry.
Manufacturers describe 4-Hydroxy Piperidine using precise data: chemical purity above 98%, moisture levels below 0.5%, and heavy metal traces under 10 ppm according to standard chemical safety protocols. Labels give the chemical formula C5H11NO, registered CAS number 5382-16-1, molecular mass near 101.15 g/mol, and UN classification if shipping internationally. These details support rigorous compliance checks in both academic and industrial research, especially where new compound authorizations require transparency. Regulatory teams make use of hazard statements relating to skin irritation and potential acute toxicity, translated across the GHS system to ensure global handling is consistent and safe.
One common route to 4-Hydroxy Piperidine involves hydrogenation of 4-piperidone under catalytic conditions, followed by a work-up to adjust pH and isolate the product. I’ve seen bench chemists adjust variables like catalyst loading and temperature to coax better yields, reflecting the stubborn streak of piperidone reduction. Sometimes direct addition of nucleophiles onto protected piperidine derivatives also builds this target in a few steps. Scaled-up processes published in technical literature show operators controlling atmospheric pressure hydrogenations and using distillation for purification; batch reactors and inline analytics help maintain product standards, a must for anyone seeking to produce material for scale-dependent applications like drug synthesis.
That hydroxyl position isn’t just for show—it gives synthetic chemists a launching pad for further chemistry. Etherification replaces the OH with larger groups to tune lipophilicity for drug-like molecules. Halogenation can turn the alcohol into a leaving group for nucleophilic substitutions, and oxidation to lactams expands access to peptidomimetic backbones. Attachment at the amine end, including N-acylation, creates advanced pharmaceutical intermediates leading to opioids, CNS stimulants, or anti-migraine compounds. In one project, a team used 4-Hydroxy Piperidine as a precursor for novel piperidine-peptide hybrids, using Mitsunobu coupling for linkage—a testament to the versatility that creative synthetic choices unlock. Each path draws on the stable platform it offers, saving time and reducing risk from breakdown products.
Depending on the supplier or the literature you’re reading, 4-Hydroxy Piperidine might turn up under names like Piperidin-4-ol or 4-Piperidinol. Catalog numbers vary by company, with Sigma-Aldrich, TCI, or Alfa Aesar all placing it under their heterocycle sections. Synonyms, including 4-Hydroxypiperidine, highlight systematic naming conventions but also the need for double-checking to avoid mix-ups, especially since subtle shifts—like N-methylation—change both the reactivity and the safety profile.
Anyone who’s handled nitrogen heterocycles knows the drill: robust gloves, eye protection, solid fume hood ventilation in case of spills or accidental vapors. 4-Hydroxy Piperidine can irritate skin, eyes, and upper respiratory tracts during handling or inhalation. MSDS sheets describe target organ impacts and urge care in storage to avoid moisture ingress and contamination, especially for gram-to-kilogram scale packages. Disposal procedures remain strict—neutralization with dilute acid for the amine, followed by incineration for bulk waste. Standard operating procedures in good manufacturing practice (GMP) settings include traceability logs so that every step—from receiving raw materials to packing finished samples—can be reconstructed in a safety audit. This attention to detail not only protects workers but also guarantees that material supplied for research or clinical trials meets rock-solid safety benchmarks.
4-Hydroxy Piperidine leaves its mark in medicinal chemistry, particularly as a core scaffold for custom small-molecule drug candidates. Blockbuster therapies for neurological conditions often include piperidine scaffolds, leveraging this platform’s properties to cross the blood-brain barrier with less metabolic breakdown. Outside pharma, this compound underpins synthesis for catalysts, polymer modifiers, and specialty dyes. Its chemical grip and adaptability find it showing up in patents as a key intermediate for high-value targets—patients needing new migraine management options, for example, indirectly benefit from the chemistry that moves through this molecule. In my lab days, we used 4-Hydroxy Piperidine as a chiral handle, exploiting selective derivatization pathways to knock out side products.
Research groups worldwide keep tuning 4-Hydroxy Piperidine’s chemistry to push boundaries in drug discovery and catalysis. Teams investigate new coupling strategies, often borrowing from green chemistry to minimize hazardous solvents and cut down on waste. One area under intense study is the asymmetric synthesis of modified piperidines, searching for ways to build libraries of drug-like heterocycles with precise control over stereochemistry. In industry, partnerships with academic groups encourage pre-competitive research, which means progress and data sharing across labs. Publications track breakthroughs in faster, higher-yielding synthetic routes, and patent filings spike each time a new therapeutic class swings into focus. From firsthand experience, bringing these developments into a scaled-up, GMP-compliant process needs close collaboration between R&D, quality control, and regulatory experts—a process built over years of incremental improvement and lessons learned from failed batches.
Toxicological studies lay bare how careful one must be with 4-Hydroxy Piperidine. Acute oral studies in rodents highlight moderate toxicity, with LD50 values prompting storage and use above standard chemical hygiene thresholds. Chronic exposure data remains limited, although vigilance around cumulative doses underpins safety protocols. Skin or eye exposure calls for prompt washing and medical attention, a fact hammered into young chemists from day one in training sessions. Environmental assessments reflect concerns about nitrogenous compounds leaching into water systems, so spill containment and waste minimization run as key themes in environmental health and safety (EHS) departments. Institutional review boards demand documentation of exposure limits, and periodic medical checkups protect people working in facilities producing or handling this compound in bulk.
Looking forward, 4-Hydroxy Piperidine holds lots of promise for next-generation therapeutic agents, especially those targeting neurodegenerative conditions or novel pain management. The toolbox for selective modification continues to expand, thanks to ongoing efforts to harness biocatalysis and flow chemistry. New patents already trace routes for more sustainable processes and fewer byproducts, putting less strain on regulatory reviewers and plant safety teams. Down the line, expect to see this compound as a stepping stone to chiral drugs, specialty polymers for advanced medical devices, and even in crop protection research. Every breakthrough puts more demands on the supply chain, making quality, traceability, and reliable safety controls more critical. Collaborative partnerships between academia, industry, and regulatory bodies will shape the responsible growth of 4-Hydroxy Piperidine in coming years, ensuring its benefits reach sectors far beyond the lab bench.
4-Hydroxy piperidine doesn’t show up in casual conversations unless you spend time in a chemistry lab or a pharmaceutical company’s research office. Still, people across the globe rely on it without even knowing its name. That’s what always struck me about chemical building blocks: their reach stretches further than most of us realize.
This compound stands out as a key ingredient for scientists working in drug development. It’s not something you’d pick up at a pharmacy by itself. Instead, it often plays a silent but crucial role behind the scenes. Companies tap into its unique structure for shaping medicines designed to fight against real-world health problems like depression, high blood pressure, and chronic pain. Without this chemical, the options on the pharmacy shelf would look very different.
Over the years, I’ve watched teams of researchers put serious effort into tracking down molecules that can make a medicine work better or come with fewer side effects. 4-Hydroxy piperidine became a common sight in chemical supply rooms for exactly this reason. It often acts as a backbone or side-group in the synthesis of pharmaceuticals. Many high-value medicines for treating schizophrenia or Parkinson’s disease can be traced back to a process involving this very compound.
Drug designers need building blocks that allow changes at specific positions on a molecule, and the hydroxy group on the piperidine ring opens doors for further modifications. This flexibility lets chemists introduce changes that improve how a drug gets absorbed, how long it stays in the body, or how targeted its effects are. Having the ability to tweak these features directly impacts the lives of patients who rely on fine-tuned therapies for chronic or life-altering conditions.
No chemical tool is perfect, and this one comes with its own set of problems. I remember working with a medicinal chemist who pointed out that access and safety can create roadblocks. Since 4-hydroxy piperidine is an intermediate in psychoactive compounds, it sometimes attracts extra regulations and supply hurdles. Quality control takes on even more weight, especially when small impurities can trigger big problems in final drug products.
Safety in the lab can’t be ignored, either. The chemical isn’t overtly dangerous in low quantities, but best practice demands protective gear and careful handling. Many scientists push for cleaner processes these days, both to safeguard people and to cut down on hazardous waste. This push for sustainability leads teams to rethink how they work with all basic building blocks—including this one.
Manufacturers have moved toward greener chemistry in recent years, investing in routes that cut down on hazardous byproducts or make use of renewable feedstocks. The pharmaceutical world has a growing responsibility to minimize environmental harm, especially as demand for drugs keeps climbing. By advancing production techniques for 4-hydroxy piperidine and related compounds, research groups and companies encourage an industry shift toward safer and cleaner labs.
Better transparency and robust supply chains can also help. As someone who’s watched supply interruptions compound problems in hospitals, I see real value in increasing global access to high-purity intermediates. This makes sure patients ultimately benefit from consistent medicines that deliver the intended results every time.
4-Hydroxy piperidine shows up in many research labs and chemical industries. Its structure, a six-membered ring with a hydroxy group, might look harmless, but experience says otherwise. This stuff isn't the sort of chemical you pick up barehanded. Inhalation, skin contact, or accidentally getting a splash in your eyes can set off some real problems—think irritation, headaches, dizziness, even more serious issues with repeated exposure.
Using common sense goes a long way, but safety in the lab demands structure. I remember a project a few years back—one junior researcher tried to skip gloves, thinking it wasn't a big deal. After a couple of rashes, we all understood that gloves and eye protection weren't just formalities.
Personal protective equipment actually shields you. Chemical-resistant gloves keep your hands safe, as 4-hydroxy piperidine doesn't take long to seep through regular fabric. Lab coats, goggles, and face shields turn minor spills into cleanup jobs rather than emergencies. I always check the fit of my goggles; a gap around the eyes offers a fast track for splashes.
Any process making use of this compound fills the space with fumes. Even a small spill leaves a sharp, biting odor lingering in the air. Good airflow, either from an up-to-date fume hood or a dedicated exhaust system, holds the line against inhaling toxic vapors. A couple of my former coworkers used to joke about “building a wind tunnel,” but they never complained about headaches after long runs. It works.
The real test happens during a spill. We once lost about 50 milliliters on the bench. Having absorbent pads, neutralizing agents, and a waste container nearby caught it fast. Quick action limits both the risk to people and the paperwork after. Spills remind everyone that seconds count. Keep emergency showers and eyewash stations ready. Most accidents come without warning; delayed reactions only make things worse.
Never stash 4-hydroxy piperidine next to oxidizing agents. Heat or sunlight speeds up reactions you don’t want to see up close. Separate chemicals in a cool, dark cabinet designed for hazardous materials. Labels fade fast under harsh lights, so keep everything updated. Believe me, reaching for an unlabeled bottle only raises stress levels.
Ask around: no lab incident ever says, “I was overprepared.” Most trouble starts with skipped training or shortcuts. Refresher sessions—demonstrating emergency procedures and reviewing safety data sheets—turn routine into habit. New staff pick up these standards by example. One of the safest labs I worked in had weekly five-minute safety drills; accidents dropped to almost zero.
Making a habit of small checks—glove fit, goggle position, fume hood airflow—saves more trouble than any single rule. Chemistry relies on trust in procedures as much as in data. Respect for 4-hydroxy piperidine starts with never underestimating the risks. That’s the difference between another smooth shift and a long night of reports and regret.
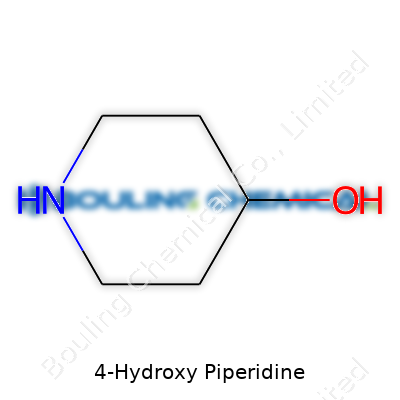
4-Hydroxy piperidine offers a good example of how chemistry shapes the world around us. It's a six-membered ring built from five carbon atoms and a single nitrogen, with each position on the ring numbered to show where different groups attach. The “4-hydroxy” part describes a single hydroxyl group connected at the fourth position on the ring. If you drew it, it would look a lot like a chair, the classic piperidine structure chemists like for its stability. One nitrogen, five carbons, and one oxygen locker give it a small but significant twist compared to regular piperidine.
Here’s how it stacks up: imagine a hexagon, with the nitrogen at the very top corner, and then count around — one, two, three, and finally, four. That fourth spot marks where the oxygen, in the form of a hydroxyl (OH) group, links up. If you look at the molecular formula, you get C5H11NO — simple, but meaningful.
I’ve come across this molecule in both academic settings and the pharmaceutical industry. The piperidine ring appears in a lot of medicines that hit pharmacy shelves. When I worked on some early-stage drug design projects, modifications at the 4-position changed the game. That tiny tweak — the hydroxy group here — can shift biological activity, boost water solubility, and change how a compound interacts with enzymes.
4-Hydroxy piperidine serves as a key building block for larger, more complex compounds. Some well-known drugs, like certain antipsychotics and pain relievers, start with piperidine rings. Adding an OH group can be the difference between a medicine working well or not even sticking around in the bloodstream long enough to help. Research has shown that these small changes often make compounds more “druggable,” more likely to get absorbed and distributed in the body the right way.
People sometimes forget that molecules like this aren’t just useful, but can also be misused. The core piperidine ring shows up in the synthesis of certain illicit drugs. Regulators and chemists have to keep an eye on the flow of these building blocks, watching for signs of diversion for nonmedical uses. On research teams, we always paid attention to where our samples came from, and followed guidelines to trace every bit of supply. Sharing best practices and transparent reporting stood out as two things that made a big difference in my experience.
Finding alternatives with similar properties but less risk of misuse represents a practical step. Teams focused on designing analogs with medical benefits that don’t play into illegal uses. Educational outreach, supported by clear scientific evidence, also helps stop molecules like 4-hydroxy piperidine from becoming problems outside of science and medicine.
Modern labs use 4-hydroxy piperidine in targeted synthesis. The hydroxy group acts as a handy spot for adding new side chains and modifying the molecule’s properties. Many researchers treat it as a launchpad for building custom molecules tuned to specific needs. Tweaking the structure at this position has led to not just new drugs, but new classes of drugs.
Collaboration between chemists, pharmacists, and regulatory bodies keeps the science moving forward without opening doors to more problems. The importance of transparency and rigorous documentation in every step can't be overstated — every bottle and shipment needs records for traceability, protecting both public health and good research.
Many chemicals can turn from harmless to hazardous in a blink if they don’t get the right attention. 4-Hydroxy Piperidine offers value for labs in pharma, chemical research, and industry work, yet its safety at workstations depends on following some fundamentals. From direct experience, storing specialty chemicals goes beyond a few words on a label; habits and choices inside a lab make all the difference. I’ve seen labs thrive with minimal incidents, and the common thread always goes back to respecting storage protocols.
A chemical’s temperament—how fast it reacts with air, light, or moisture—draws clear lines for how it should be treated. For 4-Hydroxy Piperidine, keeping it away from strong sunlight and humidity matters. If it sits out near a window or in a damp space, stability slides and the risk spikes. My lab favored cool, dark cabinets lined with clean, dry trays. A climate-controlled cabinet often keeps trouble far away. Laboratories prone to temperature swings should use refrigerators made for chemicals—not the office lunch fridge—because cross-contamination brings its own set of risks.
With time, even a snug cap gets loose. I learned early that screw-tight and chemical-resistant lids shrink panic moments. Using proper containers—preferably amber glass bottles—holds moisture and light at bay and makes cross-reactions less likely. Labels deserve as much attention as the contents. Dates, hazard signs, and concentration details mark what’s inside and what could go wrong. Neglecting labels means problems roll in for everyone, from new techs to experienced chemists hunting for reagents in a hurry. Having a clear separation of oxidizers, acids, and other reactive groups keeps things under control. Never trust memory alone—write it down and label it right.
Moisture jumps into almost any open bottle, especially as people move between spaces. Silica gel packs stacked around bottles offer a simple fix. I keep them handy, tucked beneath and around storage bottles. Tightly closed containers inside desiccators give an extra shield. Cross contact with cleaning agents or accidental spills leads to hours of cleanup and paperwork no one enjoys. Chemically segregated storage trays and shelves reduce headaches. In my own practice, dangerous mix-ups dropped once each group had color-coded bins and posted charts outlining shelf zones.
Not everyone strolling through a lab wants to follow every rule, especially on a busy afternoon. Locked cabinets with sign-out sheets manage who can handle sensitive materials. I recall one incident where an unlabeled bottle found its way onto a communal shelf—luckily, an attentive team member caught it before trouble came knocking. Trained access means fewer surprises. Digital logs and cameras may feel like overkill, but data from several industrial labs show a drop in accidental exposures after installation.
Best practice comes down to respect: for the chemical, for colleagues, for the years invested in keeping accidents rare. Routine inspections—weekly spot-checks—are no luxury. They’re how mistakes get caught before anyone pays the price. Free resources exist through chemical suppliers and safety agencies, and consulting those remains part of daily chemical stewardship. Small, clear steps keep 4-Hydroxy Piperidine in its lane—a help, not a hazard. The best labs stay curious, update protocols, and treat storage like an evolving craft, not a chore. That’s the difference between safe progress and a preventable accident.
Anyone who has spent time in research labs or chemical manufacturing knows that the quality of ingredients is never just paperwork. It determines how experiments play out, and for businesses, it marks the difference between a failed batch and a successful product. 4-Hydroxy Piperidine, a compound used in everything from pharmaceuticals to agrochemicals, comes in more than one grade or level of purity. The stakes of picking the right grade stretch far beyond routine purchasing.
During my graduate research, small impurities created big headaches. Contaminants in a core intermediate once sent weeks of synthetic work down the drain, turning a routine test into a wild guessing game. That experience taught me that not all 4-Hydroxy Piperidine on the market offers the same guarantees. Some bottles boast >99% purity, targeting the rigorous needs of the pharmaceutical world. Others offer lower specifications, typically used in preliminary experimentation or for industrial processing, where small impurities won't ruin entire runs.
Grades are not just a marketing ploy. Pharmaceutical-grade 4-Hydroxy Piperidine faces strict quality control—think analysis for heavy metals, organic residues, and any water content that might alter chemical behavior. These details increase cost, but minimize risk, especially when the final product goes inside the human body. Technical grades, not as tightly regulated, may still work for developing coatings, dyes, or as a stepping stone in multi-stage syntheses.
Manufacturers publish certificates of analysis (CoA), but interpretation calls for more than skimming tables. Methods like HPLC or NMR reveal the true level of purity and possible contaminants. A reliable supplier does not just state numbers but provides traceable data from independent testing. Inconsistent batches slow down development or force companies to remake mistakes. In my work, I have seen developers invest countless hours on troubleshooting, only to realize the source was a batch change halfway through a project.
Stricter regulations in pharmaceuticals and food have pushed suppliers to offer higher-purity 4-Hydroxy Piperidine. Academic labs, start-ups, and established firms alike now hold their suppliers to higher standards, and rightly so. FDA warning letters and international recalls usually trace back to a contamination issue that started as a “minor” difference in ingredient grade. The consequences can affect patient safety and directly influence a company’s bottom line.
Choosing the right grade starts before a purchase order goes out. Teams should evaluate supplier track records, request recent CoAs, and clarify testing methods. Risk assessment plans—now routine at larger firms—flag critical intermediates in early planning. Laboratories and production teams benefit from open communication about material sources, not just relying on paperwork. Internally, regular QC testing of every new shipment acts as a failsafe.
Suppliers willing to maintain transparency and guarantee consistency will rise as industry partners. For researchers, workers, and consumers, this attention to detail brings better, safer products and confidence in every stage of production. In the end, purity is not just a number. It is a shared responsibility embedded in every vial, every bottle, and every result.
| Names | |
| Preferred IUPAC name | piperidin-4-ol |
| Other names |
Piperidin-4-ol 4-Piperidinol 4-Hydroxypiperidine 4-hydroxy-1-piperidine 1-Piperidinol-4 |
| Pronunciation | /ˈfɔːr-haɪˈdrɒksi paɪˈpɛrɪdiːn/ |
| Identifiers | |
| CAS Number | 5382-16-1 |
| 3D model (JSmol) | ``` 3d:CCCC1CCN(CC1)O ``` |
| Beilstein Reference | 1109081 |
| ChEBI | CHEBI:37973 |
| ChEMBL | CHEMBL117999 |
| ChemSpider | 13075 |
| DrugBank | DB08156 |
| ECHA InfoCard | ECHA InfoCard: 100.030.027 |
| EC Number | 200-186-5 |
| Gmelin Reference | 82171 |
| KEGG | C14336 |
| MeSH | D010877 |
| PubChem CID | 73073 |
| RTECS number | NL4025000 |
| UNII | 7R2U8XUQ5V |
| UN number | UN2810 |
| CompTox Dashboard (EPA) | 4-hydroxy piperidine: "DTXSID8065855 |
| Properties | |
| Chemical formula | C5H11NO |
| Molar mass | 87.14 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Amine-like |
| Density | 0.995 g/mL at 25 °C (lit.) |
| Solubility in water | miscible |
| log P | 0.01 |
| Vapor pressure | 0.162 mmHg at 25°C |
| Acidity (pKa) | 9.97 |
| Basicity (pKb) | 3.84 |
| Magnetic susceptibility (χ) | -6.1×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.506 |
| Viscosity | Viscosity: 0.962 cP (20°C) |
| Dipole moment | 2.57 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 322.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -62.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4360 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. H314: Causes severe skin burns and eye damage. |
| Precautionary statements | Precautionary statements for 4-Hydroxy Piperidine: "P261, P264, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P330, P337+P313, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 1-3-0-W |
| Flash point | 99 °C |
| Autoignition temperature | 220 °C (428 °F; 493 K) |
| Lethal dose or concentration | LD50 (oral, rat): 500 mg/kg |
| LD50 (median dose) | LD50 (median dose) of 4-Hydroxy Piperidine: 500 mg/kg (rat, oral) |
| NIOSH | LM7675000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0°C to +30°C |
| Related compounds | |
| Related compounds |
Piperidine Piperidine N-oxide 2-Methylpiperidine 4-Piperidone 4-Aminopiperidine |