Chemistry’s long story with piperidine dates back over a century. Early organic researchers locked onto nitrogen-containing rings for the promise they showed in both medicinal and synthetic work. Busy synthetic labs in the mid-20th century picked up protecting groups as a kind of toolkit, and Cbz, or benzyloxycarbonyl, stands as one of the classic choices. When chemists put a formyl group on the fourth carbon of the piperidine ring and protected the nitrogen with Cbz, they built 4-Formyl-N-Cbz piperidine for synthetic flexibility. Over the years, demand for well-documented intermediates drove suppliers to offer this compound consistently for custom synthesis and pharma research. It sits in catalogs, but its story starts with industrial need, not curiosity. Researchers saw a way to step up piperidine’s value through selectivity and built new scaffolds on top of this simple modification.
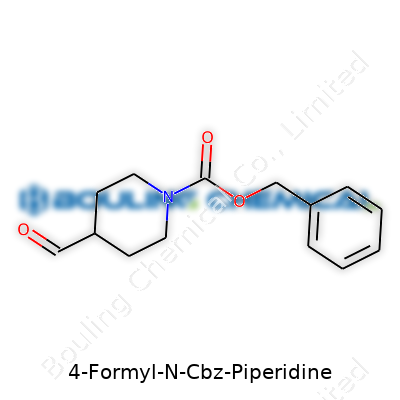
4-Formyl-N-Cbz piperidine isn’t just another line in a database. Its structure immediately hands labs a pre-packed balance of reactivity and protection. This molecule hooks a formyl group onto piperidine at the fourth spot, and covers the nitrogen with a Cbz cap. That design means you get aldehyde chemistry on one side and stable, removable protection on the other. Chemists who need a reliable aldehyde handle for condensations, reductive aminations, or ring-building work reach for this intermediate. The Cbz group lets you finish tough sequences and then reveal a free amine without tearing apart sensitive products. In my own experience, reaching for a molecule like this can make or break a project on a tight timeline.
4-Formyl-N-Cbz piperidine forms as a solid or crystalline powder. Color typically runs from white to off-white, though hints of yellow creep in if the sample spends too much time exposed. Expect a melting point in the ballpark of 70-75°C under standard conditions. The molecule refuses to stay dissolved in water, but shows good solubility in DMSO, DMF, common organics like chloroform, or ethyl acetate. Chemically, both the formyl group and Cbz protecting group can stand up to a fair variety of synthetic steps, but acid or strong hydrogenation will strip the Cbz group, and even weak bases can affect the aldehyde. Shelf life looks good under nitrogen and dry, dark storage. Around open air, especially humidity, the aldehyde may hydrate or polymerize.
Packagers sell 4-Formyl-N-Cbz piperidine by weight—usually by grams in research settings or up to kilograms in custom synthesis. Labels typically report purity as a percent, and anything above 97% by HPLC means the lot matches strict standards for both safety and performance. Trace solvents from recrystallization—let’s say ethyl acetate or methanol—get flagged on documentation. Documentation must reference CAS number 95735-65-8, offer batch-specific certificates of analysis, and include recommended handling and PPE instructions. I have seen some lots flagged for faint contamination with N-formyl isomers, but tight control in production batches keeps that rare. Anyone handling this material always checks every label, especially when purity is crucial for downstream reactions.
Getting to 4-Formyl-N-Cbz piperidine in a synthesis lab usually kicks off with N-Cbz-piperidine, ready-prepared through standard benzyloxycarbonylation of piperidine using Cbz-Cl and base. In the next step, directed lithiation at the fourth carbon with lithium diisopropylamide, often run at cryogenic temperatures, sets the stage for a carefully controlled Vilsmeier-Haack or similar formylation. Guys who’ve done this know the challenge—finding just the right balance to get good regioselectivity. Organic solvents do the heavy lifting, and thorough workup steps, including DCM washes and drying over MgSO4, give the cleanest yields. Purification comes by silica gel prep columns. You want clean NMR—not a whiff of unreacted Cbz-piperidine or monoformyl contaminants.
The real draw is the blend of reactive aldehyde and protected amine. Aldehyde groups call out for condensation with hydrazines, oximes, or even reductive amination using borohydride or cyanoborohydride. More elaborate syntheses tap into enamine chemistry or Grignard additions at C4, building all sorts of complex heterocycles. Deprotection of the Cbz group comes last, using standard conditions like hydrogenation over Pd/C under atmospheric hydrogen—or, if sensitive groups are in play, acidolysis with HBr in acetic acid. The intermediate formed here bridges classic piperidine chemistry with access to new pharmacophores. Tweaks to the Cbz group—like swapping for Boc or Fmoc—come up in projects with specialty deprotection steps. I’ve felt the difference in speed and outcome by picking just the right protective group here.
4-Formyl-N-Cbz piperidine goes by a few aliases in catalogs and papers. Look for N-benzyloxycarbonyl-4-piperidinecarboxaldehyde, N-carbobenzyloxy-4-formylpiperidine, or the more systematic 1-(benzyloxycarbonyl)-4-piperidinecarbaldehyde. Chemists working across countries spot these labels, but quality always beats branding. Some companies tuck it into their “specialty intermediates” or “protected amine-aldehydes” product categories. Always cross-check the CAS and structure, since similar names land on different scaffolds.
Whoever opens a bottle of this stuff, whether in a small academic hood or a kilo lab, needs proper gloves, goggles, and splash-resistant coat. Aldehydes will irritate skin, eyes, and mouth in short order if handled bare-handed, and N-Cbz-protected amines rarely feature in safety mishap stories—but caution still wins. Storage away from acids, bases, and oxidizers in a cool, dry place keeps risk low. The aldehyde can vapor off slightly over open containers, bumping up inhalation concerns. Proper sink disposal or waste jars for spent samples prevent nasty surprises with incompatible mixtures. Good SOP training reduces lab accidents, but reminders always matter: nobody gets careless with unknowns, especially with aldehydes in play.
Medicinal chemistry and pharma R&D lead the pack in buying and using this intermediate. Every year, industry screens hundreds of substituted piperidines for CNS, cardiovascular, and oncology candidates. The chemoselectivity and timing of deprotection make it easier to build libraries and tune activity at late stages—the kind of flexibility regulators and lead optimization teams demand. Crop science, flavor and fragrance research, and academic groups exploring new ring-forming chemistry all draw on the reliable platform this molecule brings. I’ve seen project timelines shrink dramatically after switching to pre-made, high-purity batches for parallel synthesis. The boost in speed always lands as more data, faster iterations, and less dreary troubleshooting.
Materials like this rarely stay in one lane. Universities dive into reactivity studies, mapping new carbon-carbon coupling or heterocycle assembly using this aldehyde group as a handle. Novel piperidine derivatives built off this backbone find their way into structure-activity relationship tables and patent filings every year. R&D teams in contract synthesis and pharma explore analogs that tweak the Cbz moiety for new reactivity or compatibility with emerging reactions—once obscure, but now bread and butter for high-throughput chemistry. Plenty of bioconjugation strategies bank on that stable protected amine—handy for drug-linker design or dye probes. Every season, conference posters push the boundary a little further, adding more complexity or better yields thanks to this foundational intermediate.
Comprehensive data on pure 4-Formyl-N-Cbz piperidine toxicity remains limited, which means experienced chemists treat it with respect. Its two key features raise known risks: free aldehydes can react with protein and DNA, and benzylic groups bring some metabolic concerns. Standard in vitro screens map possible mutagenicity and cytotoxicity, but so far, the parent compound’s low volatility and relatively modest dose exposure in lab settings keep it below many regulatory thresholds. Chronic exposure data remain sparse, so every material safety data sheet recommends minimizing inhalation, skin contact, and accidental spills. I’ve seen even green chemists stress the importance of good fume hoods and swift cleanup—just not worth the risk to gamble with reactive aldehyde dust or solution.
The appetite for new scaffolds in pharma and fine chemical work keeps the spotlight on protected amine intermediates. As more companies automate synthesis or pursue AI-driven drug design, demand for building blocks with reliable deprotection triggers and orthogonal handles rises each year. Environmental pushes—greener solvents, reusable protection strategies—may nudge suppliers to refine the preparation routes. Cheaper, more sustainable Cbz group installations would open the door for expanded availability, and better purification techniques cut down impurities even further. I expect more analogs and multifunctional versions on the horizon. Teams looking to combine site-specific formylation with a buffet of protecting groups chase similar compounds, broadening both chemical diversity and patent landscapes. As demand for customized and complex piperidine-based molecules climbs, 4-Formyl-N-Cbz piperidine keeps its spot in the toolkit, bridging classic bench chemistry with modern medicinal discovery.
I’ve handled all kinds of molecules in the lab, but every time I hear someone ask about the “purity” of a compound like 4-Formyl-N-Cbz Piperidine, it brings back memories of endless weigh-outs, TLC plates, and one persistent question: Do we really know what’s lurking in that vial? This particular molecule, a go-to intermediate in pharmaceutical synthesis, has a reputation for throwing curveballs if you don’t pay attention to the numbers on the label.
Years back, I tried making a batch of a piperidine-derived product, thinking 97% purity sounded solid. The reaction outcome told another story. Extra spots on TLC, odd signals on the NMR, and more headaches troubleshooting than I want to admit. Turns out, those few sneaky percent left unaccounted for meant the difference between a clean synthesis and a mess of side reactions chasing phantom yields. Purity isn’t just a number—it decides whether your process runs clean or turns into an exercise in frustration.
Most commercial suppliers advertise 4-Formyl-N-Cbz Piperidine as “≥97%” pure. Some premium vendors go higher and offer material above 98% or even HPLC-verified 99%. It’s tempting to think the difference isn’t huge, but that missing 1-2% can hide unreacted starting material, byproducts, or residual solvents. These unwanted extras don’t just dilute the potency. They might show up in analytical data, slow down purification, and in worst cases, introduce regulatory problems if used in final drug products.
Relying on a CoA (certificate of analysis) alone didn’t cut it for my team. We started running short NMR or even LC-MS scans on new batches. This first-hand data exposed more than one mislabeled or mishandled shipment over the years. A colorless powder can still hide a story—one batch fresh out of a cold truck smelled like aldehydes and staled within days. Turns out, it hadn’t been sealed properly and oxidized in transit. Running quick checks saved us time and money, not to mention peace of mind.
Handpicking suppliers with strict quality controls made a difference. Tracking lot numbers and storing sensitive materials under proper conditions cut down on headaches. Sometimes, if extra purity means spending a bit more, it’s worth checking how much trouble a lower grade could cause downstream. For extra-sensitive projects, running a quick flash column or recrystallization on arrival bumps up the purity without much trouble. Once, we pooled leftover fractions, cleaned them up, and wound up rescuing grams that would have landed in chemical waste. It taught us that knowing your material—and not assuming every white powder is the same—pays off in more ways than one.
For anyone in the thick of synthetic chemistry or scale-up, purity controls more than yield sheets—it plays into safety, speed, and lab morale. Pushing past the bare minimum cuts back on reruns. If you ever wonder if an extra percent here and there matters, ask anyone who’s filtered out a mystery impurity at the end of a long day. They’ll have stories to share. I sure do.
Storing chemicals like 4-Formyl-N-Cbz piperidine can trip up even experienced folks. At first glance, the big names and numbers on the label can look intimidating. But the details in chemical safety matter more than the fancy terminology. Years spent in labs taught me that small mistakes with storage end up burning through budgets or worse, putting people at risk.
Water and chemicals do not always get along. 4-Formyl-N-Cbz piperidine prefers to keep away from moisture, so storing it in a dry spot directly extends its shelf life. Humidity in the air, leaking around building windows, or even a carelessly left-open cap can let in enough water. Before you know it, you end up with product breakdown or gunky residue. Dessicators and tightly sealed vials do the trick—air exposure stays at a minimum. Tossing in a silica gel packet never hurts.
This compound loses quality fast if temperatures soar. Picking a cool storage area—never under sunlight, away from heat pipes, distant from ovens and sterilizers—goes a long way. Not every space qualifies. At my old research institute, leaving specialty chemicals in a supply cabinet near a window led to cloudiness in bottles and ruined samples. Consistent room temperature, or better, refrigeration around 2–8°C, helps avoid waste and guarantees stability. Still, do not place the container right by the frost vent in a fridge; a gentle, steady temp works best.
It’s easy to underestimate how much trouble ambient light or UV can cause. After a few months on a shelf, you might notice the powder shifting color. This isn’t magic—it’s often a sign of photodegradation. Opaque containers or simply storing bottles inside a closed cupboard shields the chemical from unwanted light. Even a brown glass bottle works better than anything clear or see-through.
Some compounds throw a fit in the presence of oxygen. 4-Formyl-N-Cbz piperidine isn’t supposed to be left out in the open for too long, or "just resting" with a loose lid. You want air-tight caps, parafilm, or a double-seal system if you are planning to store it for a while. We once had to throw away an expensive batch because someone “just borrowed a bit” and didn’t reseal it right.
Labeling sounds boring, but it's a lifesaver. People might joke about “mystery bottles,” but missing or unclear labeling can ruin months of work. Permanent marker with date received, structure, batch, and clear hazard signs helps everyone stay safe. If your team rotates often, this little step spares new members from confusion.
Most accidents I witnessed happened not from handling chemistry, but from simple shortcuts in storage. Run-off from a leaking fridge shelf, an unlabeled sample, or a bottle knocked over in a dark cabinet—these headaches rarely make the news but always slow down progress. Choosing the right spot, sealing containers, and logging every move in a notebook feels tedious, but lab routines built around those habits prevent bigger problems later on.
I remember my first dive into buying supplements online, and the thrill quickly faded. Browsing endless options filled with health claims, most sounded impressive, but I felt left in the dark. What really helped me make a decision was finding brands that posted their Certificate of Analysis right alongside their product info. That single piece of paper felt like a hand extended in a crowd.
A COA goes beyond a simple checklist. It gives a snapshot of what’s actually in the bottle, bag, or food you’re buying. For folks with allergies, food sensitivities, or people who just want to avoid getting ripped off, this level of transparency removes a lot of second-guessing. We’re living in a time where reports of mislabeling and contamination surface regularly. A 2023 study out of the Journal of Dietary Supplements found nearly 30% of herbal products examined contained ingredients not listed on the label. If a COA is missing, it’s easy to wonder what else might be.
A COA shouldn’t just be a digital file thrown into a folder. It often comes from an independent lab. Not all labs are created equal, but solid ones have reputations built on accuracy. They look for heavy metals, pesticides, bacterial contamination, and check purity claims. The labs aren’t infallible, but the act of testing — and showing the results — signals that someone is looking over the company’s shoulder. Think of it as having a mechanic double-check before you buy a used car.
I can’t count the number of times I walked away from a company that wouldn’t or couldn’t provide a COA when asked. I know plenty of people feel awkward contacting a customer support line, but, from experience, good companies hand over their documents gladly. The rest hit you with vague statements or try to skirt the request. That’s always a red flag. This isn’t fussiness — it’s common sense.
Sometimes, all someone wants is to be sure there aren’t any hidden surprises in what they’re taking. I’ve seen parents anxiously track down COAs for CBD products for their epileptic kids. Small farmers or health-conscious shoppers do the same with powders and herbal teas. There’s peace of mind in seeing an actual test result, not just taking a brand’s word.
There’s a debate about how much regulators should push for mandatory COAs across industries. In my view, until that scrupulous enforcement shows up, asking for a COA is a straightforward way for buyers to protect themselves. Some companies already do this as a badge of honor, setting a higher bar for everyone. Imagine a world where every supplement, food ingredient, or wellness product had a transparent paper trail.
It takes buyers leaning on sellers, and companies willing to make transparency part of their business. Instead of tiptoeing around with “proprietary information” excuses, companies should realize that open sharing builds loyal customers. Retailers could help, too, by demanding COAs from their suppliers and making them available on the shelf or online.
So, next time you’re checking out a new supplement or trendy ingredient, ask for the COA. If it’s missing, consider what that says about trust. Choosing a product shouldn’t be a shot in the dark. It should feel like a handshake between buyer and seller, backed by real proof rather than empty promises.
Chemists at any stage, from students grinding through a late-night synthesis to postdocs designing the next blockbuster molecule, deal with solubility questions almost daily. Picking the right solvent can spell the difference between a clean, overnight crystallization and a sticky, unrecoverable mess. The focus here is on 4-Formyl-N-Cbz Piperidine, a compound that pops up in many peptide and small molecule synthetic schemes. Solubility is not just a trivia matter; it drives purity, yield, and the sanity of anyone standing over the fume hood.
Take a look at the structure: a six-membered piperidine ring, a formyl group, and a carbobenzyloxy (Cbz) protecting group on the nitrogen. This skeleton delivers a balance of polar and nonpolar features. The Cbz group adds an aromatic stretch, tipping solubility toward organic solvents. That formyl group—an aldehyde—helps but doesn’t make it water-friendly. Here’s where experience in the lab kicks in: knowledge of structural fragments often predicts solvent compatibility before a drop is even added.
Years of hands-on benchwork, coupled with published data, bring up a clear shortlist for dissolving 4-Formyl-N-Cbz Piperidine. Dichloromethane (DCM) takes the lead; the compound dissolves smoothly at room temperature, making extractions and workups manageable. Ethyl acetate also acts as a reliable solvent, supporting both the needs of organic layers and common chromatography processes.
THF (tetrahydrofuran) and acetonitrile give solid performance for most reactions or analytical runs. DMSO and DMF—those polar, high-boiling workhorses—handle the compound well, although few enjoy the headaches in purification that come with their removal. For recrystallization or slow evaporation, methanol and ethanol get some mileage, though the compound’s limited water solubility rules out any direct aqueous work.
To sum it up: when planning reaction or isolation steps, stick to DCM, ethyl acetate, THF, or acetonitrile for smooth sailing. Reach for DMSO or DMF only if absolutely necessary—these often turn isolation into an afterthought. The molecule barely touches water, mainly due to the bulky Cbz group blocking polar interactions.
Plenty of chemists have faced the hassle of a poorly chosen solvent. Dumping this compound into hexanes or simple alkanes will do nothing but provide a cloudy suspension—speaking from a time I tried to encourage precipitation too quickly and only made a mess. On the other hand, using overly polar solvents can drive unexpected side reactions, especially given the reactive aldehyde. Imagine losing valuable product in a wash step just because ethyl acetate was swapped for something less suitable like pure methanol.
Solubility tables in databases are helpful but rarely capture the mix of conditions met in real synthetic work: pressure built up, temperature swings, and the realities of batch reactions. Teaching careful solvent testing—actually coaxing a small sample of the compound into various solvents and watching what happens—serves better than any digital prediction. Simple logbooks, updated with each run, offer a dose of practicality often missing from digital protocols.
Wider sharing of empirical results, not just through formal articles but in lab groups or forums, speeds up the learning curve. Few want to waste time repeating someone else’s solubility struggles. After countless rounds of trial and error, knowing the expected outcome before even starting a reaction lets the chemistry flow smoother and the bench stay cleaner. Solvent choice may feel like a minor detail, but for chemists making or refining 4-Formyl-N-Cbz Piperidine, it’s one detail that can’t be overlooked.
Anyone spending time in a synthetic chemistry lab will quickly bump into names like 4-Formyl-N-Cbz piperidine. It’s not a household compound, but if you’ve ever had a hand in research around pharmaceutical compounds or advanced materials, you might notice this molecule open up a lot of doors. The structure alone—a piperidine ring with both formyl and carbobenzyloxy (Cbz) groups—screams “intermediate” to anyone used to organic synthesis. This compound doesn’t end up in a bottle at your pharmacy, but without it, some key steps in drug creation hit a dead end.
Let’s say you work with medicinal chemists. The effort that goes into tweaking and optimizing a molecule’s skeleton takes time, skill, and building blocks like 4-Formyl-N-Cbz piperidine. It’s the kind of intermediate you pull off the shelf to build up more complex heterocyclic drugs. The piperdine core alone shows up all over the pharma industry—think antipsychotics, antivirals, and plenty of others. Add a formyl group and there’s a reactive spot, perfect for further changes. The Cbz-protected nitrogen keeps the amine stable under tough conditions, letting chemists get creative with the remaining parts of the molecule.
A lot of new drugs start their journey as a screen of variations. You make a hundred, a thousand analogs, trying to find one that works best. Having a compound like 4-Formyl-N-Cbz piperidine in the toolkit speeds up that search. I've seen teams run full-on campaigns, switching the bits and pieces that hang off the core, finding new leads for painkillers, anti-cancer drugs, or treatments for neurological disease. The value lies in its flexibility—something pharmaceutical chemists always crave.
Peptide chemistry has its tricks, too. Many researchers learn the hard way that peptides love to fall apart if left unprotected. The Cbz group in this molecule steps in as armor. It rides through the harsh steps of synthesis, letting scientists build more complex chains before clipping it away. Every person who’s run peptide coupling on a tight deadline knows the stress of unstable side groups or messy side reactions. Buying a ready-made synthon like this lets you skip headaches and move on to purifying your product.
Some labs use it to jump into custom ligands and enzyme inhibitors. Chemistry aimed at blocking proteins often needs a piperidine twist here or a formyl tweak there, and this intermediate brings both in one package. If you needed to design a probe for biology or want a handle for coupling reactions, this gives you a shortcut. Speed matters in drug discovery, and anything that cuts the total number of steps keeps costs down—something every research manager tracks closely.
There's always a flip-side. Whenever I’ve seen big labs run large projects using intermediates like this, the quality of the source becomes a real issue. Impurities can ruin a whole string of reactions. Strict quality control, reliable suppliers, and tight shipping standards solve most of this, though sometimes labs hedge their bets by making it themselves in-house. This eats time, but control stays in your hands.
Green chemistry edges in, too. Scaling up for pilot production poses a big question—Can reaction steps work in larger batches without toxic byproducts? Teams keeping an eye on waste and regulatory scrutiny put energy into finding cleaner routes and safer conditions.
In the end, 4-Formyl-N-Cbz piperidine keeps showing up in the toolkit—reliable, flexible, and ready for whatever the next project demands.
| Names | |
| Preferred IUPAC name | (1-benzylcarbonyloxy)piperidine-4-carbaldehyde |
| Other names |
N-Cbz-4-piperidinecarboxaldehyde 4-Formyl-1-benzyloxycarbonylpiperidine 4-Formyl-N-(benzyloxycarbonyl)piperidine |
| Pronunciation | /ˈfɔːr.mɪl ɛn siː bɛdˈziː pɪˈpɛrɪdiːn/ |
| Identifiers | |
| CAS Number | 1416998-39-6 |
| Beilstein Reference | 120398-76-3 |
| ChEBI | CHEBI:141415 |
| ChEMBL | CHEMBL3705802 |
| ChemSpider | 10949240 |
| DrugBank | DB08343 |
| ECHA InfoCard | 07-02-07-02213-1 |
| EC Number | SCHEMBL1055539 |
| Gmelin Reference | Gmelin66343 |
| KEGG | C19831 |
| MeSH | D017220 |
| PubChem CID | 102273787 |
| RTECS number | DH2308000 |
| UNII | UOV872JH1U |
| UN number | UN3272 |
| CompTox Dashboard (EPA) | DTXSID5060349 |
| Properties | |
| Chemical formula | C14H17NO3 |
| Molar mass | 263.32 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.15 g/cm3 |
| Solubility in water | Insoluble |
| log P | 1.90 |
| Acidity (pKa) | 13.4 |
| Basicity (pKb) | pKb = 3.24 |
| Magnetic susceptibility (χ) | -72.44·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.579 |
| Viscosity | Viscous oil |
| Dipole moment | 3.1725 Debye |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | C1CN(CCC1C=O)C(=O)OCC2=CC=CC=C2 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | Health: 2, Flammability: 1, Instability: 0, Special: – |
| Flash point | 179.5 °C |
| NIOSH | NA |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10-50 mg |
| Related compounds | |
| Related compounds |
4-Formylpiperidine N-Cbz piperidine 4-Formyl-N-Boc piperidine N-Boc piperidine 4-Formylpiperidine hydrochloride N-Cbz-4-hydroxypiperidine |