People have been tinkering with morpholine chemistry for over a century, chasing solutions in rubber, pharmaceuticals, dyes, and more. 4-Ethylmorpholine came onto the radar as chemists searched for tailored amines that boost process control and product outcomes. Its roots draw from the broader morpholine family, which started breaking ground in synthetic chemistry labs after World War II, a period awash in new organics and a drive for industrial growth. Factories once flirted with simple morpholine, but as processes got more exacting, researchers saw value in adding that ethyl group, opening up new performance traits needed for advanced applications in coatings, catalysis, and synthesis, especially as precision chemistry ramped up in the 1970s and 80s.
4-Ethylmorpholine doesn’t grab headlines, yet it plays a strong supporting role behind the scenes of chemical production. It’s a clear, colorless liquid, but in the same way a good stagehand works quietly, its chemical backbone shapes processes without a lot of fanfare. Suppliers typically ship it in drums or jerrycans, focusing on purity levels since trace byproducts can send reaction results sideways, especially in demanding pharmaceutical settings. The product usually carries over 99% purity with limited water content, and solid labeling helps keep storage hazards manageable in crowded warehouses.
Anyone working with 4-ethylmorpholine notices its distinct, ammoniacal odor. The liquid boils at about 157°C and weighs just under a kilogram per liter. Its notable polarity and the presence of both an amine and an ether group open plenty of doors in synthesis. Miscibility leans heavily toward both water and organic solvents, which has always made this chemical useful for folks who don’t want extra solubilizing steps. Watch for vapor pressure at room temperature—about 2 mmHg at 20°C. Left unguarded, vapors can sneak into workspaces, so ventilation is a must.
A typical drum of 4-ethylmorpholine features a label carved with data from a gas chromatograph—chromatogram peaks mapping out purity, signals on residual water, and flags for unwanted byproducts like acetaldehyde. Chemical indexers reference CAS Number 100-74-3. UN codes draw attention to its flammability, placing storage and transport under the watchful eye of international chemical safety standards. Labeling should flag its irritant nature and recommend gloves, goggles, and careful ventilation, especially where splash risks live around transfer pumps and bottling lines.
Lab workers have chased efficient routes to 4-ethylmorpholine for decades. Most setups react ethylene oxide with ethylamine in the presence of acid catalysts, often under pressure. Early syntheses lacked the temperature controls we use today, leading to variable yields and gnarly byproducts. Modern processes squeeze better conversion by dialing in stoichiometry and scrubbing oxygen-sensitive intermediates. Engineers use closed systems, sometimes recycling unreacted feedstocks. It’s a telling sign of how economics and safety nudge manufacturers to relentlessly improve, chasing higher yields with smaller footprints.
Like its cousins, 4-ethylmorpholine brings versatility to the table. Its tertiary amine group responds nimbly in alkylation or acylation reactions. Beyond running as a base in organic synthesis, the molecule also serves as a building block—developers attach functional groups at the ring nitrogen or the ethyl tail, tossing out custom ligands, intermediates, or even surfactants. Derivatization with halides, isocyanates, or nitrosating agents springs open new doors for drug design, resin improvement, or even corrosion inhibition, especially in custom-engineered performance chemicals.
Curious shoppers track this compound under multiple aliases: N-Ethylmorpholine, 4-ethyl-tetrahydro-1,4-oxazine, or just plain 4-ethylmorpholine. Industry catalogs list it under its CAS number for precision, but it often finds its way into trade formulations under specialty names, reflecting its role as a reactive intermediate or niche catalyst. Patent literature might mention it tucked inside process flows or as a keystone ingredient in proprietary recipes.
Working with 4-ethylmorpholine brings the same headaches and cautions as other small-amine compounds. Skin contact stings, and the vapors irritate eyes and lungs. Safety training drills into muscle memory the need for gloves, goggles, and, for larger transfers, chemical splash aprons. Emergency showers and eyewash stations earn their keep in labs that handle it frequently. Regulatory groups in Europe, the U.S., and Asia have all placed the compound on lists for hazard communication, requiring clear labeling under GHS rules. Storage keeps to cool, dry spots, and facilities run explosion-proof pumps, since vapor buildup could invite flash fires if left unchecked.
Few outside the field realize how a compound like 4-ethylmorpholine weaves into so many sectors. In drug discovery, it tweaks pH or serves as a building block for experimental APIs. The coatings industry leans on it for its stabilizing punch in epoxy and urethane resins, feeding demand for durable, tough-wearing finishes. Some water treatment processes count on it for specialty surfactancy, while the oil industry occasionally leans on it for scavenging acidic leftovers. Academic researchers put it under the microscope in catalysis or to support the search for novel ligands. Practical chemists keep it on hand as a workhorse base, especially where a strong, non-nucleophilic touch is needed.
R&D often shines brightest where curiosity and need collide. Teams in universities and chemical companies keep nudging 4-ethylmorpholine into new reactions, often pushing for asymmetric catalysis or greener processes. Some groups are curious about swapping functional groups to tune solubility and reactivity, especially for custom pharmaceuticals or fine chemicals. Startups sometimes chase closed-loop production, aiming to cut waste and re-use solvents, seeing economic and environmental sense at once. As green chemistry draws more advocates, there’s steady pressure to find less hazardous alternatives or to improve worker safety through better enclosures and process automation.
Anyone who’s handled 4-ethylmorpholine knows safety data sheets pull few punches: inhalation or skin exposure leads to burning or stinging, and animal data points to moderate acute toxicity. Chronic exposure data remains thin, but most research warns operators not to drop their guard. Regulators in Europe and the U.S. demand workplace exposure limits, and some environmental groups nudge for closer study, especially around aquatic toxicity and bioaccumulation. Researchers pressing for data transparency argue for deeper dives into long-term health impacts. Some animal assays suggest potential nervous system effects if mishandled, so facilities keep spill kits and neutralizers at arm’s reach.
New uses for 4-ethylmorpholine keep cropping up in both technical literature and patent filings. Demand hasn’t spiked like a trendy specialty chemical, but its steady role in coatings, catalysis, and as a niche pharma building block keeps it afloat. If green chemistry gets another boost, makers may pivot to improved production routes, recycling, or higher-purity formulations. Digital process control offers another step up for safer, more efficient manufacturing. Replacement interest could grow, especially for high-toxicity applications, so R&D will likely keep chasing safer, less-irritating analogs. Anyone invested in sustainable manufacturing will want to keep an eye on regulatory changes and clean-process breakthroughs, as those are the trends shaping tomorrow’s specialty chemical landscape.
Most people walk right past the world of specialty chemicals without a second glance. 4-Ethylmorpholine, with its odd-sounding name, rarely finds itself discussed at the dinner table. It isn't a household staple, but plenty of industries rely on it. For those unfamiliar, 4-Ethylmorpholine takes the form of a colorless liquid, and comes loaded with a strong, fishy odor — the sort that makes you want to twist the cap back on in a hurry.
Stop by a polyurethanes factory and you’ll probably find a drum or two of 4-Ethylmorpholine. Companies add this compound as a catalyst to help speed up chemical reactions during the making of polyurethane foams. Foam feels like part of everyday life—from car seats and sofa cushions to insulation panels. The people working to deliver better, longer-lasting foam often count on 4-Ethylmorpholine for quicker, more reliable curing.
On the pharmaceutical side, this chemical sometimes helps with synthesizing active ingredients. Medicinal chemists often hunt for nitrogen-containing rings when building new drugs; 4-Ethylmorpholine, with its morpholine structure, works as a helper or building block in those setups. While you won’t spot it listed as an ingredient on your pill bottle, its role shows up behind the scenes, allowing complex drugs to come together in an efficient way.
Beyond pharmaceuticals and flexible foams, some folks in the agrochemical business draw on 4-Ethylmorpholine during pesticide production. Every field of chemistry has a knack for repurposing compounds, and this one pops up whenever someone needs a tertiary amine that creates just the right reaction conditions.
If there’s a reason to give this chemical a wide berth, it stems from its safety profile. Breathing in 4-Ethylmorpholine can leave workers feeling dizzy and irritated, both in the lungs and in the eyes. Skin contact won’t do anyone favors either. People who handle the stuff suit up in gloves and goggles, and follow strict ventilation rules.
Lately, workplaces aim to swap in less hazardous chemicals whenever possible, but with tight budgets and technical demands, change rarely comes overnight. What matters is common sense: training, personal protective equipment, and proper procedures keep accidents rare.
Chemicals like 4-Ethylmorpholine don’t stay put. Spills threaten water supplies and disrupt aquatic life. The problem often boils down to waste disposal—a leaky drum, or a shortcut down the drain, and pretty soon the ecosystem pays the price. As someone who spent time in a manufacturing plant, the memory of safety drills and “spill kits” still lingers. Success came down to discipline and vigilance, not fancy gadgets.
The industry faces a steady push from environmental regulators. Tracking, labeling, and documentation take priority. Factories adopt closed-loop systems to capture fumes and prevent leaks. While bureaucracy gets a bad rap, those paper trails force companies to think twice before cutting corners.
Plenty of brainpower focuses on finding replacements. High school science students learn to mix and match reactants, and the same curiosity powers industry labs trying for safer, greener substitutes. Switching away from 4-Ethylmorpholine places less stress on everyone from workers to wildlife.
It isn’t just about swapping one molecule for another—reformulating products can take years, and some alternatives fail under pressure. Until scientists and engineers make more headway, living with chemicals like 4-Ethylmorpholine remains a reality. For now, smart handling and a healthy dose of caution keep the wheels turning.
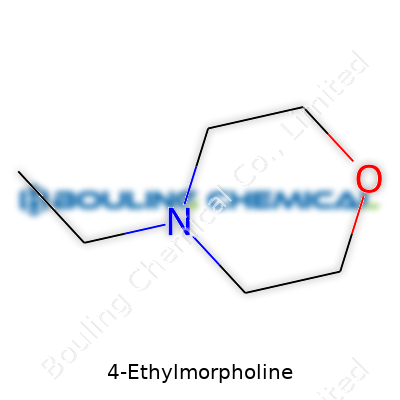
Chemistry names have a knack for sounding complex, yet the meaning hides in plain sight. Take 4-Ethylmorpholine. The chemical formula for this compound reads C6H13NO. Imagine a regular morpholine ring—a six-membered structure, part nitrogen, part oxygen. Now tack an ethyl group (that two-carbon fragment, –CH2CH3) onto the fourth carbon. That's 4-Ethylmorpholine, nothing mysterious when you look at the skeleton: a ring, two elements with a two-carbon tail sticking out.
Anyone skimming past the diagrams may miss the weight of small tweaks like an ethyl group. Adding it changes how the molecule moves and interacts, much like shoelaces make all the difference between boots and slippers. I remember in grad school, how our organic chemistry professor pounded this idea into us: one new attachment, and the whole behavior of the compound shifts. For 4-Ethylmorpholine, this tweak nudges the compound into fresh territory—solvent ability, pH adjuster, reaction medium. Even small groups like ethyls nudge the boiling point upwards and alter how the molecule dissolves in water or organic solvents.
The world of 4-Ethylmorpholine shows up far from a textbook. This compound slides easily into labs and manufacturing, especially when there's a need to stabilize a mixture or nudge a pH number in aqueous media. Its basicity helps snap reactions to attention. Working with polyurethane foams, chemists reach for it to speed up the reaction between isocyanates and polyols. There's no glamour behind the bench, but a molecule like this keeps processes running smooth.
All chemicals come with trade-offs. 4-Ethylmorpholine isn't just a gentle helper—it can irritate skin and eyes on contact. Breathing in vapors brings headaches or more. I learned quick in the lab that gloves and goggles aren't optional, and ventilation isn't just a box to tick. The Safety Data Sheet speaks plain language: watch your exposure, handle with care, store away from acids. It’s the same story with loads of chemicals. Still, it pushes the question: can smarter handling or structure tweaks give us safer yet effective tools?
Keeping chemistry safe sits on more than warnings or checklists. Real change grows out of training, clear labeling, honest risk assessment. For folks working around 4-Ethylmorpholine, simple steps—good gloves, sealed containers, spill kits nearby—make a difference. Researchers keep digging for gentler analogs, probing if removing or swapping out an ethyl could strip out some harshness without losing the chemical punch. The answer often arrives slowly but methodically: share data, test alternatives, question any shortcut.
Chemistry reflects creativity and caution working side by side. 4-Ethylmorpholine only stands out through small design changes—a tweak on a simple ring. It proves that progress isn’t always about reinventing the wheel but sometimes about refining existing tools, step by step. Handling with respect, searching for even better answers, and remembering that each little group or bond in a molecule can echo throughout a whole industrial system. That’s real-world chemistry, lived every day from benchtop to factory floor.
4-Ethylmorpholine sounds pretty complicated, but at its core, it’s a colorless, oily liquid used in various chemical syntheses and as a solvent. Having worked around chemical stockrooms, this chemical rarely stands out in memory, but that doesn't mean it’s safe to treat with casual neglect. Anyone who’s handled a few of these amines knows you don’t just shove them onto a random shelf and hope they behave. It’s not just about ticking boxes on a safety list — a little awareness avoids headaches or worse.
There’s no secret sauce for storing hazardous chemicals, but 4-Ethylmorpholine has its quirks. Temperatures above room level push up pressure inside its container, which can lead to leaks or ruptures. I’ve seen forgotten cans under a sunny window balloon up like a party trick. Ambient storage below 25°C slows everything down, from vapor pressure to chemical degradation. If the facility uses warehouse space with patchy AC, it’s worth investing in a proper chemical fridge or at least a cool, shaded cabinet.
This compound doesn’t mix well with strong oxidizers or acids. It’s tempting to pack chemicals close together to save space, but one spill and a poorly placed jug of acid can spark a reaction nobody wants to clean up. I once had to help with a fuming mess because someone nested amines next to a jug of peroxide. Safety sheets and color-coded bins exist for a reason.
Glass and some plastics handle 4-Ethylmorpholine just fine, but squeezing the last penny by reusing old, contaminated bottles leads to cross-reactions. It might smell bad, which is one clue, but labeling every bottle with the purchase date and contents tags helps anyone doing inventory avoid blind guesswork.
Seal everything tightly. Even small leaks add up, especially with a liquid that evaporates easily. The stuff’s vapors irritate the nose and eyes, something you notice even with a short whiff during transfer. One trick I learned is to dab a little petroleum jelly around a bottle’s threads; it makes a better seal and saves the label from dissolving vapors. Ventilated storage, like cabinets with built-in exhausts, stops vapor buildup and keeps workplace air safer. No one wants to find out their headache came from the storeroom.
Anyone who’s poured out bulk bottles of 4-Ethylmorpholine knows the splash hazards. Gloves, goggles, and lab coats aren't for show, and a face shield helps with big pours. An eyewash station and a spill kit nearby make it possible to react, not just panic, if something goes wrong. I’ve found that training newcomers with a mock spill drill gets better results than lecturing just from the manual. You tend to remember the stinging itch of a simulated splash longer than any warning.
For transfer in large quantities, local exhaust and closed transfer systems can keep the process clean. Improvised setups often lead to leaks or overfills, so it pays to use proper dispensing pumps or containers designed for sticky amines. Walking home in clothes soaked with vapor isn’t something anyone volunteers for. Handling rules protect everyone — not just the person doing the job right then, but the next person coming into the workspace.
Label every bottle clearly, store in a cool, ventilated spot, separate from incompatible chemicals, and use personal protective gear during transfers. Double-check seals and make note of any odors or discolorations — I always trusted my nose over a half-worn expiration label. Proper spill management tools, regular inventory checks, and straightforward safety training go a lot further than assuming old habits will be enough. Staying on top of these details turns what might be a risky material into just another manageable part of the lab routine.
Ask a chemist and they might rattle off long-winded safety data sheets, but for those of us outside the lab, 4-ethylmorpholine sounds mysterious and technical. Yet, this chemical plays a backstage role in making coatings, dyes, and even specialty cleaners. Industry uses it for its ability to dissolve or stabilize other chemicals. That prosaic role doesn’t mean it’s safe to brush off, though.
I’ve seen plenty of chemical labels that flash warnings most people ignore. With 4-ethylmorpholine, there’s a reason to pause. Breathing its vapors, even in small amounts, can irritate your nose, throat, and lungs. Eyes sting, skin reddens, and long-term exposure raises more serious questions. Animal studies have flagged kidney and liver effects when the chemical is handled carelessly or with spotty ventilation.
The Occupational Safety and Health Administration and the European Chemicals Agency both set strict limits on airborne exposure—those agencies don’t tighten rules without reason. I keep seeing factory workers where gloves and goggles only come out if a supervisor walks by. That’s a recipe for trouble, since this stuff can seep through skin after a few careless spills.
Some folks think hazard means just immediate poisoning. 4-ethylmorpholine also burns easily, giving off toxic smoke and gases. Fires in warehouses or processing plants can quickly turn local air nasty for nearby communities. In past incidents, firefighters faced headaches and nausea even through protective masks. The environment takes a hit, too. Spills leach into soil and mess with groundwater, sticking around for a while.
Water treatment experts know this headache. They deal with residues that hang around, complicating cleanup drives. And yet plants sit near waterways because of cheap land or easy transport, making the risk of accidents something regular folks might never realize—until they catch a whiff or spot a plume.
There’s no shortage of reports about chemical exposure accidents. Yet some companies still cut corners. They buy lower-quality safety gear or skip regular hazard training. From experience, I can say people relax after a few months working with the same chemical, but all it takes is one splash or spill to bring everything back into sharp focus.
Most of the time, good ventilation, gloves, goggles, and tight seals on containers keep things under control. The trouble pops up when a shipment leaks, or a worker rushes a cleanup. Adding leak sensors or upgrading to automation for risky transfers costs money, but it saves a lot more in the long run—both in fines and in medical bills.
It's tempting to leave chemical safety to experts. I’d say anyone who stores, ships, or works with 4-ethylmorpholine should rethink convenience over caution. Even managers and logistics folks ought to know the basics of exposure limits and emergency procedures. Swapping hazardous chemicals for safer alternatives helps most, though substitutes aren’t always readily available.
For communities near chemical plants, pressing for transparency matters. Public reports about usage and spills give residents a chance to ask hard questions and push for better safeguards. I’ve talked to union stewards and safety officers who stress the power of a safety-first culture: nobody’s invincible, and nobody wants chemicals turning up in their backyard, either.
People often overlook the true impact of chemical purity on a project. Having spent years around industrial labs and chemical suppliers, the difference between a 98% and a 99% pure sample is anything but a mere decimal point. In the case of 4-ethylmorpholine, purity usually sits in the 98% to 99% range. That number isn’t just for a catalog; even a tiny impurity can create big headaches. If your end product relies on this compound, a lower grade could introduce side reactions, waste resources, or contaminate pricey batches of pharmaceuticals, coatings, or polymers.
It’s easy to assume every supplier works to the same standard, but experience shows otherwise. Some manufacturers, especially those catering to specialty or pharmaceutical clients, will list traceable impurities right on the Certificate of Analysis. On the other hand, suppliers focused on bulk markets could only guarantee “98% min.” without much else in terms of accountability. For anyone who must hit strict regulatory targets or produce something consistent batch after batch, those details matter more than marketing claims.
Most people don’t give chemical packaging a second thought until a mishap occurs. Years working with solvents and intermediates taught me to favor drums with robust seals. 4-Ethylmorpholine, being a liquid, typically arrives in blue steel drums or HDPE containers. Think of 25 kg drums for the smaller end, which works well in R&D or pilot plant settings. Bulk buyers opt for 200 kg drums, and the occasional IBC tote for real volume.
The packaging isn’t about presentation; it determines shelf life, spill risk, and workplace safety. I remember the stench when a poorly sealed drum leaked in summer storage—it wasn’t just an unpleasant smell, it turned a manageable project into a hazardous waste headache. Containers need to handle the chemical’s volatility and basicity, or you deal with rust, corrosion, and eventual contamination. Unlined steel doesn’t cut it. HDPE survives, but can warp with rough handling. Suppliers worth their salt run leak tests and provide tamper-evident seals.
There’s a rush to drive down cost, especially for routine intermediates like 4-ethylmorpholine. I’ve seen companies cut corners only to get stuck with returns, reworks, and customer complaints—costing more in the end. Regulatory agencies now track every input more closely. A minor impurity flagged on a pharmaceutical audit can delay products by weeks. Even for coatings, inconsistent supply leads to visible quality defects. The industry learned, sometimes painfully, that reliable purity and safe packaging actually protect profitability.
If sourcing this compound, don’t just hunt for the lowest price or fastest delivery. Good procurement asks for that extra bit of information: a recent Certificate of Analysis, third-party testing options, and clear product handling guidelines. It’s one thing to read “colorless liquid, 99%,” and another to open a drum filled with yellowed residue and mystery odors.
Reliable suppliers keep detailed documentation and answer questions about stability, compatibility, and shelf life. They invest in packaging that survives a six-month sea voyage, not just a quick truck ride across town. They’ll share experiences from similar clients, not just repeat technical specs. Choosing well here saves trouble all the way down the supply chain. Every day in the lab or plant confirms this wisdom: An extra half-hour vetting the supplier protects months of smooth production.
| Names | |
| Preferred IUPAC name | 4-Ethylmorpholine |
| Other names |
4-Ethylmorpholine N-Ethylmorpholine |
| Pronunciation | /ˌfɔːrˌiːθɪlˈmɔːrfəliːn/ |
| Identifiers | |
| CAS Number | '100-74-3' |
| Beilstein Reference | 1209249 |
| ChEBI | CHEBI:57830 |
| ChEMBL | CHEMBL16300 |
| ChemSpider | 11130 |
| DrugBank | DB14089 |
| ECHA InfoCard | 100.011.198 |
| EC Number | 202-970-7 |
| Gmelin Reference | 8286 |
| KEGG | C02345 |
| MeSH | D021483 |
| PubChem CID | 12015 |
| RTECS number | QS9625000 |
| UNII | 403N1J8A42 |
| UN number | UN3456 |
| Properties | |
| Chemical formula | C6H13NO |
| Molar mass | 116.18 g/mol |
| Appearance | Colourless liquid |
| Odor | amine-like |
| Density | 0.921 g/mL at 25 °C (lit.) |
| Solubility in water | miscible |
| log P | 0.49 |
| Vapor pressure | 0.9 mmHg (at 25 °C) |
| Acidity (pKa) | 8.54 |
| Basicity (pKb) | 5.15 |
| Magnetic susceptibility (χ) | -6.38×10^-6 cm³/mol |
| Refractive index (nD) | 1.429 |
| Viscosity | 1.9 cP (20°C) |
| Dipole moment | 3.06 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 319.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -322.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4647.1 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes severe skin burns and eye damage. Causes serious eye damage. Toxic to aquatic life with long lasting effects. |
| Precautionary statements | P210, P261, P264, P271, P280, P301+P312, P304+P340, P305+P351+P338, P311, P321, P330, P337+P313, P370+P378, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | 32 °C (90 °F; 305 K) - closed cup |
| Autoignition temperature | 195 °C |
| Explosive limits | 2.2–11% |
| Lethal dose or concentration | LD50 oral rat 2150 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 2150 mg/kg |
| NIOSH | SY8575000 |
| PEL (Permissible) | PEL: 100 ppm (400 mg/m³) |
| REL (Recommended) | 3 ppm |
| IDLH (Immediate danger) | 100 ppm |
| Related compounds | |
| Related compounds |
Morpholine N-Methylmorpholine N-Ethylmorpholine 4-Methylmorpholine Piperidine |