Chemists first encountered 4-Chloro-N-Methylpiperidine decades ago while searching for attainable ways to tweak the piperidine ring. The demand for functionalized heterocycles in medicine and agrochemicals grew sharply after the 1960s. During this time, research groups started testing small substitutions on piperidine structures. Chloro-derivatives stood out, not only for their reactivity but also for paving new paths in synthesis. Over time, companies in the United States, Europe, and Japan added this compound to their portfolios, responding to calls from medicinal chemists and process engineers. Each laboratory put a spin on its production methods, shaping the compound’s journey from a niche research molecule to one widely available for custom synthesis or scale-up.
Anyone handling fine chemicals for pharmaceuticals or materials knows about piperidine derivatives. 4-Chloro-N-Methylpiperidine carves out a special place, offering a balance between utility and manageable hazard. Labs use this colorless-to-pale-yellow liquid for a range of reactions where a piperidine base is needed, but where the backbone must stay chemically active for later steps. The compound’s lone nitrogen and the strategically placed chlorine give chemists much-needed leverage for functionalization. As technology improves, suppliers offer this molecule with documents showing purity, impurity profile, and batch consistency, aiming to satisfy researchers and technical buyers alike.
The molecule weighs about 133 grams per mole. Its boiling point sits just above 185°C, so distillation or removal from complex mixtures isn’t too challenging. The density falls near 1.04 g/cm³, and the liquid shows low solubility in water, yet dissolves easily in organic solvents like ether and chloroform. Chemically, the 4-chloro group tightens up the ring and makes the nitrogen less basic compared to simple piperidine, yet it leaves the molecule reactive where needed — a feature frequently exploited in cross-coupling or displacement reactions. Handling the compound in air requires some care: the odor is sharp and unpleasant, a reminder to use well-ventilated hoods.
Commercial bottles arrive labeled with UN numbers, hazard pictograms, and typical GHS warnings. Most suppliers guarantee purity above 98%, but residual water or secondary amines sometimes creep in unless the material is freshly distilled. Buyers demand batch chromatograms, elemental analysis, and certificate of analysis. Regulatory details include EU REACH registration, US TSCA listing, and shipment with the right shipping documents. Labels warn: keep out of sunlight, store below 25°C, seal tightly after opening, and use appropriate gloves and goggles.
Producers use a couple of go-to routes for synthesis. One common approach starts from N-methylpiperidine, reacting it with chlorine sources or thionyl chloride under controlled conditions. Some choose a route involving N-methylpiperidone, which gets converted using phosphorus-based reagents, then neutralized to free the base. The selection depends on purity targets, lab safety, and waste treatment capabilities. Factory chemists optimize for yield and environmental footprint, cycling mother liquors and recycling process water to cut down on waste.
The compound’s biggest draw comes from its readiness to undergo nucleophilic substitution. That chlorine at position four hops off in favor of oxygen, nitrogen, or sulfur atoms, enabling the creation of diverse intermediates for drug and dye synthesis. Some groups push the reactivity even further, using metal-catalyzed processes to tack on aryl or alkyl units, turning the molecule into a launchpad for numerous downstream products. Experienced chemists watch temperature and solvent choice carefully, since the ring can sometimes open or rearrange under strong conditions, producing a tangled mess.
In industry and academic settings, this material carries various names. Synonyms include 4-Chloro-1-methylpiperidine, N-Methyl-4-chloropiperidine, and sometimes methyl-4-chloropiperidine. International buyers see slightly altered spellings, though CAS number 104-88-1 identifies the compound for procurement everywhere. Trade names may differ, particularly with specialty chemical houses or catalog suppliers each offering branded versions, but the core structure stays unchanged.
Working with 4-chloro-N-methylpiperidine involves risks tied to skin absorption, inhalation, and accidental ingestion. Most labs require goggles, nitrile gloves, and the use of an appropriately rated fume hood. In my own workspaces, standard procedure goes beyond the minimum: chemical-specific training, clear spill kits, and emergency eyewash stations nearby. The compound irritates eyes and respiratory passages, so sealed containers and careful transfer matter even for small-scale jobs. Waste must go to halogenated solvent streams, and the compound shouldn’t go down the drain or into regular trash.
The reach of this molecule spreads from pharmaceutical intermediates to research on agrochemicals and specialty polymers. Medicinal chemistry teams rely on it to create sophisticated drugs where an active amine group or its derivatives drive biological activity. The molecule paves the way for antipsychotics, antivirals, or pesticides that need just the right balance of hydrophobicity and reactivity. In other corners of industry, R&D teams explore its use as a catalyst modifier or as a building block for advanced materials where nitrogen-based rings impart flexibility or adhesion.
Development labs explore both new ways to make this compound cheaper and safer, and new applications in molecular design. A decade ago, few process flowsheets included steps to recover and reuse solvent, but now the pressure to lower emissions drives process innovation. Green chemistry gets a stronger voice: researchers try to swap toxic reagents with safer ones and adopt continuous flow reactors—a big move for pilot or commercial scale projects. Presentations at conferences highlight not only synthetic successes but case studies from companies struggling with scale-up, contamination, or regulatory hurdles.
Toxicologists have run a variety of tests, uncovering moderate acute toxicity in rodents and significant irritation to mucous membranes. Skin and eye exposure lead to lasting discomfort, and inhalation of vapors presents respiratory hazards. Chronic exposure data remain sparse, so firms keep exposure as low as possible. Teams continue screening for long-term carcinogenic or mutagenic effects, since regulatory bodies keep tightening standards for chemical intermediates found in pharmaceuticals and ag-chem products. Every material safety data sheet includes stark warnings, and new findings trigger updates to protocols and labeling.
Chemical manufacturers and startups push to expand the usefulness of 4-chloro-N-methylpiperidine. The future points toward new routes that reduce waste and unearth value in by-products. Machine learning-driven retrosynthesis promises to spark creative new transformations, potentially opening up downstream products now considered too expensive or complex to make. Regulatory authorities urge companies to adopt cleaner, closed processes, and customer demand for product traceability and lower environmental impact steers development. If cost drops and supply chains stabilize, more research groups could treat it as a standard arylation or amination precursor, fueling the next wave of active pharmaceutical ingredients and specialty materials.
Talk to anyone with a background in organic chemistry and they’ll have some stories about molecules that look plain but have serious influence. 4-Chloro-N-Methylpiperidine fits that bill. This compound pops up more often than folks realize, acting as a solid building block for inventing new materials, drugs, and lab tools.
Back in college, I spent long nights in the lab watching people tinker with piperidine rings, sometimes hunting for tiny changes that could flip a molecule from useless into something lifesaving. Chemists add a methyl or chlorine to a basic ring, not for kicks, but to control how the next step in a reaction runs. The 4-chloro and N-methyl tweak give this compound extra flexibility, letting it help in building complicated molecules fast.
A lot of drugs need piperidine derivatives. Think painkillers, antipsychotics, and antiviral agents. 4-Chloro-N-methylpiperidine helps create those core structures. Its value comes from the way it steers chemical reactions, guiding the creation of strong bonds without the drama of unwanted side products. For researchers looking to design fresh molecules, that reliability helps speed up the race from lab test to trial.
Farmers depend on new pesticides and herbicides to handle tougher seasons and pests that seem smarter every year. The journey from concept to a product on store shelves travels through labs where compounds like this play a vital role. Using chlorinated piperidines, agrochemical makers try to outsmart resistant weeds and insects. 4-Chloro-N-methylpiperidine’s specific structure lets scientists shape highly targeted compounds, working toward formulas that work harder on pests but pose fewer headaches for crops and the people growing them.
Not all uses focus on health or agriculture. Industrial labs blend 4-chloro-N-methylpiperidine into specialty chemicals that end up in coatings, dyes, and even electronic parts. I once shadowed a team exploring ways to tweak liquid crystal materials for phone and TV screens, and they turned to piperidine derivatives for their stability and ability to handle high temperatures. The versatility of this compound means it can be the missing piece in making chemical reactions more efficient and predictable.
Anytime a molecule turns up in pharmaceuticals, pesticides, and specialty industries, there’s bound to be some baggage. 4-Chloro-N-methylpiperidine falls into a group where careful oversight matters. Some intermediates like this get watched because they have the potential to act in unexpected or even harmful ways outside the lab. Handling rests on strict safety rules, including proper storage and disposal, so that research benefits don’t come with environmental or health risks.
For regular folks, the chain ends before they ever see or touch pure 4-chloro-N-methylpiperidine. But its influence runs through the products beneath our feet on the farm fields, inside medicine cabinets, and even our electronics. With smart policies and focus on worker safety, chemists can keep pushing innovation without inviting trouble.
4-Chloro-N-methylpiperidine won’t grab headlines, but advances in what it helps create often will. Seeing it through the lens of discovery, I respect its quiet importance — from shortening drug pipelines to making crops stronger and technology more reliable, all routed through that unremarkable-looking piperidine ring. Chemistry always hinges on pieces like this connecting big ideas to practical reality.
At the heart of many pharmaceuticals and chemical research projects, small tweaks to a compound’s structure can lead to big changes in how it behaves. One such example is 4-Chloro-N-Methylpiperidine. Essentially, this chemical features a piperidine ring, which is a simple six-membered structure made up of five carbon atoms and a single nitrogen atom. Now, add a twist: on the fourth carbon, a chlorine atom is attached. On the nitrogen, a methyl group throws its hat in the ring. Drawn out, you see the piperidine scaffold holding a bulky methyl and a chlorine, giving it a unique personality among simple amines.
This compound shows just how flexible organic chemistry can get. The nitrogen in the ring gives it a basic character. Tacking on a methyl makes it a little more stubborn, and that chlorine throws in new chemical possibilities. This isn't just academic trivia. Shifts in chemical makeup like this can completely change how a compound works in practice, influencing everything from reactivity to the way it interacts with biological systems.
Chemists have been relying on piperidine rings for decades. They show up in everything from medicines to agricultural chemicals and even some flavors. Changing just one atom, as seen in 4-Chloro-N-Methylpiperidine, opens doors for entirely new research avenues. Throwing a chlorine on the fourth position increases its potential for building more complex molecules, especially through reactions like nucleophilic substitution. The methyl group on the nitrogen makes it more selective, providing a route for chemists to explore beta-blockers, antihistamines, or even new catalysts.
In laboratories, tweaking the structure of compounds isn’t just about making “something new” – it’s about tuning them to meet specific needs. 4-Chloro-N-Methylpiperidine, because of its arrangement, can act as an intermediate for many drugs under development.
Just because a compound has exciting potential doesn’t mean it gets a free pass. Chlorinated organic compounds deserve careful handling. Exposure to the smallest amounts can cause irritation or worse problems if the safety rules aren’t followed. Responsible research depends on making sure everyone working with these chemicals has access to safety information and proper training. Up-to-date safety data sheets should always be within reach, and the right protective gear can’t be left in the closet.
Waste management also becomes a priority. No one wants chlorinated residues entering the water supply or the air. Laboratories have a duty to collect, label, and dispose of chemical waste according to tough regulations. This responsibility keeps communities safe and shows science can be both innovative and accountable.
Research doesn’t slow down. Each modification to a familiar chemical ring shapes the path for tomorrow’s discoveries. 4-Chloro-N-Methylpiperidine stands out as a tool for unlocking new reactions and building better pharmaceuticals. Efforts to continually improve lab safety, streamline synthetic methods, and share findings openly let everyone benefit from the next breakthrough. The story of this compound is still being written in labs around the world.
4-Chloro-N-methylpiperidine belongs to the piperidine class, a group of chemicals that pop up in both labs and larger industrial settings. Its structure shares traits with other alkylated piperidines, but the addition of a chlorine atom brings its own quirks in terms of reactivity and handling concerns. That small change means it swings between being an asset in synthesis and a challenge for anyone tasked with keeping it both safe and stable on the shelf.
Anyone who’s handled chlorinated amines will know the importance of storage environments. I can’t count how many times I’ve walked into research labs and seen opened bottles or loosely capped flasks left sitting for weeks. This is a shortcut to instability. 4-Chloro-N-methylpiperidine reacts with moisture in the air, and over time, this can kick-start slow decomposition. Storing it in a tightly sealed container—preferably amber glass—reduces light exposure, but more importantly, minimizes the air and humidity entering the container. If you keep it around water or acids, unwanted reactions might creep in, altering both purity and yield during synthesis.
In my experience, flammable, low-boiling chemicals draw plenty of concern, but folks often overlook chlorinated amines like this one. The volatility isn’t pronounced, but gradual evaporation still happens, especially if left unchecked in warmer rooms. Cold rooms or dedicated chemical refrigerators, kept at 2–8°C, significantly keep volatility and degradation at bay. For sample quantities held long-term, the freezer (at -20°C) makes a difference in cutting down peroxide and impurity formation.
The chemical stability of 4-Chloro-N-methylpiperidine isn’t just a textbook concern. From available material safety data and published synthetic procedures, heat, light, and exposure to open air all chip away at its shelf life. In labs where temperature spikes and humidity go unchecked, I’ve seen even sealed bottles begin to lose clarity and take on faint odors—clear signals of slow breakdown.
Studies addressing amine hydrochloride salts show increased stability, thanks to the absence of free amine and lower reactivity. But for 4-Chloro-N-methylpiperidine in its base form, minimizing its contact with oxidizing agents and acids remains vital. Over time, breakdown products can gum up reactions or produce toxic byproducts. A simple label with storage dates and regular inspection—checking for changes in color or texture—offers early warnings if things start to shift.
Common problems such as leaky lids, sharing flasks between projects, and prolonged bench exposure increase risk. A solid solution: use dedicated, well-labeled bottles with screw caps and desiccant packs inside chemical storage cabinets, away from direct sunlight. For anyone shipping or storing across facilities, keeping the compound in its salt form almost always proves safer.
Working in teams, I’ve found the best method is peer reminders—quick checks during routine inspections catch problems before they turn into costly replacements or ruined batches. Clear logs tracking opening dates and storage conditions help spot trends before they escalate. It’s not just about following protocol, but about making sure every synthesis down the line starts with pure, trustworthy material.
Anyone managing 4-Chloro-N-methylpiperidine needs to treat its storage as essential—not as an afterthought. Skipping steps in routine checks leads to wasted resources and unpredictable chemistry results. Reliable storage—cool, dry, airtight—paired with regular review sets up both lab teams and industrial processes for success. Controlled handling is just as important as any technical parameter in a reaction scheme; get that right, and the chemistry works as planned.
4-Chloro-N-methylpiperidine ranks as a specialty chemical used by researchers and in industrial synthesis. Its backbone sits in the piperidine family, compounds that find homes in drug development and advanced manufacturing. The chemical itself is not a household term. Only a slice of the population—chemists, lab techs, and those in the pharmaceutical sector—find themselves near it.
Handling this chemical raises flags for anyone who knows their way around a lab. The piperidine ring bears a reputation for reactivity. Adding a chlorine atom changes the equation, turning it into a substance that can irritate the skin, eyes, and lungs. Splash this compound or breathe in its vapors and discomfort won’t take long to follow. Splashes risk chemical burns, while airborne particles may bring on headaches, dizziness, or worse with enough contact.
Material safety data shows risks like toxicity if digested or inhaled. Shortness of breath, fatigue, or nausea may signal exposure. Piperidine derivatives have drawn plenty of attention for neural effects, so it’s smart to avoid unnecessary contact. Those cooking up experiments with this substance take precautions dictated by government safety boards, including gloves, goggles, and ventilation. Ignoring these steps can invite long-term damage, not just a bad day at the bench.
Combustion throws another curveball. 4-Chloro-N-methylpiperidine brings flammability to the table. Sparks or open flames spell trouble in the lab or at storage sites. Once burning, the fumes released can do more harm than the original substance, since toxic gases like hydrogen chloride or nitrogen oxides join the mix. Quick and careful cleanup of any spills becomes a duty—protecting coworkers and the workplace from accidents and lingering vapor.
Disposal presents a second problem. Pouring waste down the drain lets toxic chemicals slip into local waterways. Over time, small doses carried out by many hands can stack up. Aquatic species already face pressure from farm runoff and plastics. Adding novel, synthetic chemicals complicates the puzzle. Fines and penalties mount for labs or factories caught dumping chemicals irresponsibly. Personal experience as a college chemistry lab tech taught me that the quiet choice—disposing of waste in the right containers—costs pennies, and ducking responsibility costs careers.
Most chemical risk boils down to how it’s handled. Governance in the U.S., Europe, and much of Asia sets limits for storage and transport of hazardous chemicals. Training sessions and audits hold people accountable. Most mistakes spring from ignoring lessons and safety protocols designed to keep both users and environments safe. Neighbors downwind or downstream from research campuses rely on strict adherence to the rules as much as the scientists indoors.
Chemicals like 4-chloro-N-methylpiperidine belong in controlled spaces, not the garage or garden shed. The lessons from industrial accidents echo for decades: one misstep and a legacy of harm lingers in soil and water. Pushing for greener alternatives or redesigning industrial processes to cut out hazardous intermediates makes sense for everyone—factory worker, consumer, and anyone walking near a stream.
Anyone working with molecules like 4-chloro-N-methylpiperidine holds both power and responsibility. While innovation in chemistry grows industries and cures diseases, one shortcut in safety can erase that progress. Lessons picked up in the real world—by seeing burned gloves or emptied eyewash stations—stick with you. These reminders create a strong case for careful handling, training, and disposal. Safe practices turn chemicals from a source of dread into powerful tools.
High purity always gets top billing for any fine chemical, and 4-Chloro-N-Methylpiperidine falls into that camp. Most labs and industrial outfits won’t touch material unless it clocks in above 98%. Reputable suppliers usually offer chemicals in purities like 98%, 99%, or sometimes even higher. This isn’t just a detail for chemists obsessed with numbers. The presence of lower-grade material can drag down your experiment or process, introduce side reactions, and even render analytical work pointless. If a synthetic route relies on a high-quality amine, even a fraction of a percent’s worth of a contaminant sometimes throws off everything downstream: yields drop, impurities creep in, and re-work costs time you can’t get back.
Getting burned by low-purity material teaches a hard lesson. I once watched a team burn through weeks of lab work only to learn that a batch of a key piperidine derivative contained traces of water and unknown byproducts. Cleanup ran so long that the opportunity window closed before the results came in. Since then, everyone asks for full certificates of analysis and keeps a standing relationship with trusted vendors. That’s how important purity has become for research and production.
Chemical markets no longer serve just multinational corporations. Individual labs, custom synthesis providers, and mid-sized manufacturers often need unique volumes. For 4-Chloro-N-Methylpiperidine, most suppliers sell it starting at modest quantities—little glass bottles or polymer jugs containing 5 grams, 25 grams, or 100 grams. For scale-up work or manufacturing, you see packaging in 500-gram, 1-kilogram, and up to 20-kilogram containers. Bulk orders get drum packaging at 25 kg, 50 kg, or even 200 kg sizes.
These aren’t just arbitrary measures. Glass ampules or small plastic bottles work for the bench, since they reduce exposure to air and moisture. Jugs or drums, on the other hand, become crucial in handling, storage, and shipping for large quantities. Every chemist dreads opening a fresh container only to smell decomposition or see discoloration and water beads—signs the closure failed, or the bulk was divided for retail by someone lacking basic care about air sensitivity or hygroscopicity. Real-world stories float around about labs receiving piperidines in poorly sealed polybags; by the time a researcher opens it, the contents have already absorbed moisture and turned yellowish. Money goes down the drain.
Before buying, labs and companies can request lot-specific data and ask how each batch was handled, filled, and shipped. This is not being fussy—it’s cost-effective diligence. Regulatory requirements are tightening around chemical shipping too, affecting what sizes can go by air or must go by truck. Smaller bottles sometimes escape certain hazardous material regulations, speeding up delivery. That sort of trade-off matters when a research deadline looms or production needs ramp up without delay.
Suppliers who back up their material with full documentation, lot traceability, and robust packaging handle most setbacks before they turn into disruptions. If more buyers demanded transparency on both purity and packaging, the market would see fewer problems with stockouts, failed reactions, or hazardous leaks. I’ve seen teams cut downtime dramatically just by building long-term relationships with vendors who spell out exactly what’s shipping out the door.
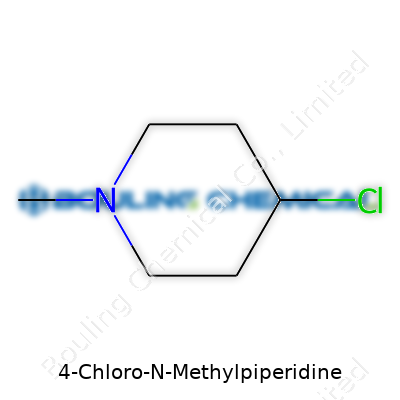
| Names | |
| Preferred IUPAC name | 1-Methyl-4-chloropiperidine |
| Other names |
4-Chloro-1-methylpiperidine N-Methyl-4-chloropiperidine 4-Chloro-N-methyl-piperidine |
| Pronunciation | /ˈklɔːroʊ ɛn ˈmɛθəl paɪˈpɛrɪdiːn/ |
| Identifiers | |
| CAS Number | 10250-27-8 |
| 3D model (JSmol) | `CCCC1(CCN(C1)C)Cl` |
| Beilstein Reference | 70744 |
| ChEBI | CHEBI:187130 |
| ChEMBL | CHEMBL490330 |
| ChemSpider | 13293313 |
| DrugBank | DB08875 |
| ECHA InfoCard | ECHA InfoCard: 100_010_572 |
| EC Number | 273-382-2 |
| Gmelin Reference | 145215 |
| KEGG | C19276 |
| MeSH | D015637 |
| PubChem CID | 12423082 |
| RTECS number | TM6475000 |
| UNII | W87QM5V3Y8 |
| UN number | UN3431 |
| Properties | |
| Chemical formula | C6H12ClN |
| Molar mass | 133.62 g/mol |
| Appearance | Colorless liquid |
| Odor | ammonia-like |
| Density | 0.978 g/mL |
| Solubility in water | soluble |
| log P | 1.71 |
| Vapor pressure | 0.33 mmHg (25 °C) |
| Acidity (pKa) | pKa = 11.2 |
| Basicity (pKb) | 2.81 |
| Magnetic susceptibility (χ) | -74.33·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.482 |
| Viscosity | 1.54 cP (20°C) |
| Dipole moment | 3.09 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 336.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4811.7 kJ/mol |
| Pharmacology | |
| ATC code | N05CM21 |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS06 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H314 |
| Precautionary statements | P210, P261, P271, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 63°C |
| Autoignition temperature | 270 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat): 290 mg/kg |
| LD50 (median dose) | LD50 (median dose) = "290 mg/kg (rat, oral) |
| NIOSH | IP1225000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 ppm |
| Related compounds | |
| Related compounds |
N-Methylpiperidine 4-Chloropiperidine N-Methyl-4-piperidone Piperidine 4-Chloro-N-ethylpiperidine |