Looking back, the story behind 4-Chloro-1-Methylpiperidinium Chloride shows how advances in basic organic chemistry end up supporting real industry. Laboratories first began making piperidinium derivatives in the mid-20th century because of their promise for pharmaceuticals, catalysis, and industrial processing. Tinkering with chlorination and methylation of piperidine produced a family of new salts, among which the 4-chloro-1-methyl version earned a niche for its specialized reactivity and accessibility. Corporate and academic chemists took note, filing patents or publishing stepwise methods, especially in the 1970s and 80s, pointing to uses in both research and component manufacturing. Over time, production grew more consistent, yielding a compound now routinely cited in both fine chemical catalogues and relevant technical papers—a marker of its reliability for modern applications.
In daily practice, 4-Chloro-1-Methylpiperidinium Chloride comes across as a specialized quaternary ammonium salt. Most often, it lands in labs and plant supply rooms in tightly sealed containers, either as crystalline powder or a fine-grained solid. The product holds value for pharmaceutical chemists, agrochemical developers, and synthetic methodologists who rely on piperidine backbone reactivity. It's no bulk commodity, but instead fits the profile of a chemical ordered deliberately, guided by a reference in a patent or a synthetic scheme.
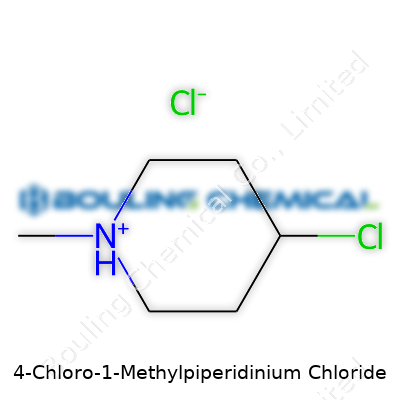
Most of the time, 4-Chloro-1-Methylpiperidinium Chloride appears as a white to off-white solid, showing stability under ambient conditions if kept dry and out of UV. Structural analysis points to a six-membered piperidinium ring with a chlorine atom locked at the 4-position, methyl at the nitrogen. The chloride balances the charge, making it highly soluble in water and most polar solvents—something handy for practical workups. Melting point falls within a moderate range, avoiding volatility, yet the salt will decompose at higher heat without igniting. Its ionic nature means it doesn't play nicely with strong oxidizers, and standard incompatibilities with strong bases or reducing agents show up in storage instructions for labs.
Reputable suppliers usually guarantee a purity above 98% for research-grade batches. Typical lot certificates specify residual moisture, content of related impurities, and a statement on heavy metals—important for anyone preparing injectable pharmaceuticals or working with sensitive catalytic systems. Containers must carry not just the chemical name and formula but proper risk phrases, hazard pictograms, and detailed handling instructions. Regulatory traceability remains critical in international shipping, so every shipment includes batch numbers and supplier records for recall or quality audits.
Preparation starts with piperidine, a common building block in industrial organic chemistry. Methylation at the nitrogen, often accomplished via methyl iodide or dimethyl sulfate, forms 1-methylpiperidine, which then gets chlorinated at the 4-position using reagents like N-chlorosuccinimide or chlorine gas under controlled conditions. Neutralization with hydrochloric acid aids in precipitation, yielding the chloride salt. Each of these steps calls for careful adjustment of stoichiometry and temperature, with solvent choices ranging from basic alcohols to dichloromethane. After the reaction, purification by recrystallization or chromatography brings out material of sufficient purity for advanced syntheses or pharmacological screening.
Chemists appreciate 4-Chloro-1-Methylpiperidinium Chloride not only as an end product but as a reactive template for modifications. The chlorine atom attached to the piperidine ring opens the door for nucleophilic substitution, letting other groups swap in, from amines to complex nucleophiles. Methylation at nitrogen locks in quaternary ammonium behavior, making this salt resistant to simple dealkylation and imparting unique solubility. In the presence of base, the molecule can undergo further ring chemistry, while the chloride counterion makes it compatible with a broad array of salt metathesis reactions. This flexibility sets the compound apart for those building libraries of piperidine derivatives or engineering new ion transport agents.
Depending on catalog and region, the compound circulates under various aliases: 4-Chloro-1-methylpiperidine hydrochloride, 4-chloro-N-methylpiperidinium chloride, and other systematic variations reflect the differing conventions in chemical nomenclature. These alternative names often lead to confusion, especially for newcomers, so cross-referencing CAS numbers and standardized identifiers remains common good practice in the industry and academia.
Safety remains front of mind for any operation involving chlorinated ammonium salts. Direct contact with the solid or concentrated solutions can irritate skin and eyes, while dust generation needs to be minimized due to the risk of inhalation. Comprehensive SDS documentation from suppliers gives stepwise guidance on handling, personal protection, and accident response. Labs install fume extraction or work within enclosures. For disposal, established protocols call for dilution and neutralization, avoiding ordinary drain disposal. Those making scale-ups pay real attention to local environmental and workplace safety laws, making sure all containers and waste streams are traced and treated by licensed handlers.
Most of the real-world use for 4-Chloro-1-Methylpiperidinium Chloride comes from pharmaceutical synthesis, where the piperidine scaffold features in multiple drug classes, from antipsychotics to analgesics. The unique structure plays a role in generating new ligand candidates for screening or as an intermediate in stepwise alkaloid structural mimicry. Agrochemistry takes advantage of the compound’s reactivity to fashion new pesticides and herbicidal agents. Sometimes, you will see it used in the study of ionic transport across membranes, owing to its quaternary charge and solubility properties. Companies developing new materials turn to this intermediate for tailoring polymer and resin modification processes.
Researchers continue to publish new articles on methods for synthesizing, functionalizing, or deploying 4-Chloro-1-Methylpiperidinium Chloride, seeking ways to improve selectivity or lower production costs. In academic settings, teams are using derivatives to build niche molecular probes, explore interactions with biological targets, or illuminate SAR (structure-activity relationships). Patent filings signal attempts to integrate this compound into new generations of bioactive molecules or specialty catalysts, while conferences feature updates on yield, stereoselectivity, and green chemistry protocols rooted in this family.
Investigators test 4-Chloro-1-Methylpiperidinium Chloride for acute and chronic toxicity in mammals, fish, and test cultures. Results point to moderate toxicity on direct exposure, yet no broad evidence for bioaccumulation in soil or aquatic environments if handled with standard safety. Rodent studies indicate possible cholinergic or neurological effects at high doses, so regulatory guidance recommends restricting occupational exposure and verifying against known allergenicity or carcinogenicity data sets. Toxicology labs keep refining their benchmarks, feeding data back to manufacturers and regulatory bodies.
Looking ahead, the future for 4-Chloro-1-Methylpiperidinium Chloride seems tied to both regulatory forces and innovation pipelines. On one hand, greener synthetic methods—including catalytic or electrochemical chlorination—are under exploration to cut down on waste and lower the environmental footprint. On the other, the rise in targeted therapies and designer chemicals in both life sciences and materials means that tailored piperidine derivatives continue gaining ground. Better toxicity data will boost acceptance, while improvements in supply chain traceability will make it easier for new markets to access the compound safely. The path forward depends on practical breakthroughs in preparation, more reliable hazard management, and early identification of unique activity profiles in both disease treatment and safer agrochemical design.
4-Chloro-1-Methylpiperidinium Chloride sounds like a tongue twister from a chemistry class, but its name pops up where chemical reactions shape the products people use everyday. Those who work in pharmaceutical, chemical, and materials research see this compound as more than just a long name; it plays a crucial part in making certain reactions possible. Folks outside these areas might not realize just how much their lives are shaped by these behind-the-scenes chemicals.
The main use for 4-Chloro-1-Methylpiperidinium Chloride lies in organic synthesis, especially as a so-called “quaternary ammonium salt.” In plain terms, it acts as a phase transfer catalyst. That means it helps two chemicals that normally wouldn’t mix come together and react. This job is critical when there’s oil and water in a reaction flask, and the scientist wants both sides to talk to each other.
From my experience working with chemists who need to boost yields without turning the lab into a mess, this approach saves both time and resources. Phase transfer catalysis gets reactions moving faster and more efficiently because it encourages compounds from two otherwise stubborn phases to mingle. The impact reaches all sorts of products, from basic plastics to high-value antibiotics.
In the world of drug development, a process left halfway finished costs time, money, and sometimes lives. Medicinal chemistry groups rely on compounds like this one to help manufacturers squeeze out better yields during synthesis, meaning fewer resources get wasted. An active pharmaceutical ingredient that costs less to make because of better catalysis can end up in the hands of more patients around the world.
I’ve watched teams struggle with sluggish reactions. They’d stand over the workbench looking frustrated, wishing for a clean workaround. Adding 4-Chloro-1-Methylpiperidinium Chloride often served as a fix, giving that nudge needed to break through bottlenecks. Factories that scale up production also see the benefits, because smoother reactions mean less energy needed and fewer toxic byproducts to clean up later.
With all the upside, there’s a responsibility to handle these chemicals safely. Quaternary ammonium salts can be hazardous without proper protocols, both for workers and the local environment. Companies that value safety make sure their staff stays trained, and I’ve seen firsthand how real-world practice and strict adherence to safety data sheets make a difference. Labs improve air flow, double-check storage, and keep protective gear ready so no one gets caught off guard.
Disposal raises another set of questions. Throwing away chemical residues can create pollution if managed poorly. Some places have adopted greener practices—such as recycling or breaking down leftover chloride-containing compounds before release—to lessen downstream impact. Government agencies keep an eye on these industries for a reason, and active participation in regulatory compliance protects everyone.
Research into safer, more sustainable alternatives for phase transfer catalysis keeps moving forward. Green chemistry initiatives call on companies to rethink which chemicals push reactions along. Meanwhile, 4-Chloro-1-Methylpiperidinium Chloride continues to find its place in both old and new synthetic routes, as long as folks keep balancing performance with safety and impact on the world around us.
Handling specialty chemicals like 4-Chloro-1-Methylpiperidinium Chloride falls outside the comfort zone of many labs and warehouses. A misstep leads to ruined product or, worse, safety hazards for the people around. My own time managing a research lab hammered home the value of not getting lax with storage routines—especially when chemicals like this one can shift from harmless to hazardous with just a bit of moisture or heat.
4-Chloro-1-Methylpiperidinium Chloride pulls moisture in like a magnet. Humid air spoils the substance’s purity and can trigger slow breakdowns inside the storage bottle. Ordinary humidity, even in a regular storeroom, brings problems. It demands a tightly sealed container. I always found that glass containers with reliable Teflon-lined caps (not metal, not cheap plastic) worked best, since glass avoids unwanted reactions and seals grind tight. After a few bad runs, I also started keeping containers in a desiccator cabinet or with a pouch of silica gel right in the chemical’s jar.
There’s a problem some folks run into, stacking chemicals by the hundreds in one stuffy storage closet. Temperatures climb out of the comfort zone, sometimes hitting the high 20s or more. This chloride likes a steady setting below 25°C, and lower wouldn’t hurt. Even a regular household refrigerator beats a rack in a warm storeroom, especially through a summer heatwave. But freezing isn’t needed and can cause the solid to clump. Sticking to room temperature is safe, but nothing beats the control you get in a temperature-monitored cabinet.
Forget windows and overhead lighting. The stuff doesn’t hold up well with sunlight or UV. I always chose amber bottles or tucked clear containers into boxes. It’s easy to overlook, but even a few weeks by a sunlit window creates brownish impurities—and these used to be the bane of our quality control team.
It’s tempting to save time on simple tasks. I learned through hard-won mistakes that scruffy, half-peeled labels cause confusion fast. Each container needs a fresh, clear label with full compound name, hazard warnings, and date received. This isn’t just safe—regulators expect it. And it keeps less-experienced team members from grabbing the wrong substance for their experiments.
No storage solution stands complete without a plan for accidents. If this chloride hits skin or eyes, it stings and may cause lasting problems. A spill kit with absorbent pads and goggles hung by the door makes a clear difference. Training matters too: I learned the hard way that not everyone remembers protocol during a minor spill unless drills are run once or twice a year.
Safe storage of 4-Chloro-1-Methylpiperidinium Chloride isn’t rocket science, but it rewards attention to small details. Sealed, dry, cool, and dark shelves prevent costly losses and keep people safe. It comes down to giving chemicals the respect they demand, each and every day.
4-Chloro-1-Methylpiperidinium Chloride isn't a household name. Its presence mostly turns up in more technical chemical settings, outside everyday products. The first time I came across it, I was helping a friend sort through some lab materials before disposal. At a glance, its long name made me wonder: just how risky is this stuff, really?
Digging through resources from the European Chemicals Agency (ECHA) and the National Center for Biotechnology Information (NCBI), I found this substance classified as an organic chloride salt. It doesn’t have the mountain of toxicology reports found for more notorious chemicals—no huge red flags pop up in public hazard databases. That often means it hasn’t flooded workplaces or the market, which somewhat limits large-scale accident data. Still, being a piperidinium compound with chlorine attached means it deserves a closer look.
Based on chemical relatives, some risk pops up. Chlorinated piperidine-based substances sometimes act as irritants if breathed in or if they hit bare skin or eyes. If you swallow enough, you risk stomach pain and nausea—that’s not terribly different from other specialty lab salts. The chloride component often leads to extra caution due to possible reactivity, especially in the case of accidental mixing or heating. Signs point to short-term (acute) issues over long-term or cancer-causing effects, but testing leaves gaps.
I talked to a couple of chemists who handle similar compounds. Gloves, lab coats, goggles—nothing fancy, just regular, old-fashioned personal protective equipment. They told me they treat it like many other low-volume synthesis chemicals: mind the bottle caps, don’t sniff the stuff, double-check disposal. That matches up with general industry guidance for specialty salts. One lab manager summed it up: “If you play it smart, it doesn’t ruin your day.”
One worry is what happens once it leaves the flask. Like other organic chlorides, improper disposal raises risks for aquatic animals if it hits drains or soils in high enough doses. Most labs neutralize or pack it out with their hazardous waste, rather than sending it down the sink. Current research doesn’t track massive use or spills, but agencies recommend keeping it away from waterways, just in case. Precaution, not panic.
Most folks outside a chemistry lab never bump into this chemical, but there’s a lesson here. Even rare chemicals need clear safety sheets and disposal plans, not just silent shelf space. If you end up with a mystery chemical, always check with poison control or your environment health office—better safe than sorry. Educators ought to run tight inventories and make sure all handlers know what’s what, especially for backup chemicals and supply chain swaps. Staying honest about unknowns and not fudging hazard guesses builds trust with both workers and the public.
4-Chloro-1-Methylpiperidinium Chloride may not grab headlines, yet treating it with the same respect as other specialized lab chemicals covers both safety and environmental bases. No heroics, no shortcuts. Just gloves, goggles, and clear rules for whatever ends up in the bottle.
4-Chloro-1-Methylpiperidinium Chloride has the formula C6H14Cl2N. In simple terms, it carries six carbon atoms, fourteen hydrogens, two chlorines, and a nitrogen. The base of this compound comes from piperidine—a six-membered ring best known in synthetic and medicinal chemistry. Slap a methyl onto the nitrogen, a chlorine onto the fourth carbon, and bring in a chloride ion to balance the charge. That formula gives clarity for anyone working in a lab or checking a database. When you add up the atomic weights, the molecular weight lands at about 186.09 g/mol. That number holds weight: it decides how much you scale up for reactions, how you balance equations, and how you figure dosing or purity for your research goals.
In university labs, I watched students hit roadblocks because they misread a compound’s structure or overlooked counterions. For chemists and medical researchers, small oversights grow into big problems. Having the right formula and molecular mass helps limit those mistakes. You end up wasting fewer resources, save time, and keep your work reliable. Academic journals and regulatory agencies keep a close watch on accuracy for a reason: one transcription error or swapped molecular weight distorts the science down the line—skewed results, unsuccessful syntheses, dosing errors, or worse.
This compound, by design, fits into organic synthesis as a building block or intermediate. Some researchers lean on it for ionic liquids analysis. Others test its properties for new reactions, especially in pharmaceutical labs combing through lead candidates. I’ve seen fine chemical suppliers get requests for this salt, not because of widespread commercial applications, but because even compounds with a short ingredient list can pivot projects if the right lab gets a hold of them. Consistent nomenclature and stable supply chains don't just help the person ordering—these foster higher standards in chemical and pharmaceutical fields. Researchers keep innovation going by sharing clear information.
Sifting through online catalogs or chemical databases brings confusion when compounds with similar names carry different counterions or structural tweaks. Having the formula C6H14Cl2N spelled out avoids confusion with other piperidinium salts. Even seasoned scientists benefit from double-checking reference data: suppliers can list batch-to-batch discrepancies, or change naming conventions without much warning. Mixing these up in a synth, or assuming the presence of a different ion, risks setbacks. I learned early on to pair the molecular weight with spectra and real analytical data: honest cross-checking beats assumptions every time. That discipline plays a big role in safety, cost, and reproducibility.
Science and medicine demand trust—between colleagues, between vendors and labs, and in the published record. Suppliers and academic teams with a track record for accuracy rarely face legal or compliance issues. Embracing hard facts—like the chemical formula and true molecular weight—goes beyond good paperwork. It supports the foundation researchers depend on for future therapies, materials, and discoveries. In my experience, keeping a sharp eye on these basics always pays off, whether you’re troubleshooting a failed synthesis or training new hands at the bench.
Handling chemical shipments can throw up challenges. Regulations shift across regions, and a substance flagged as safe for open sale in one country can attract scrutiny at customs elsewhere. 4-Chloro-1-methylpiperidinium chloride isn't a household name, but laboratories and manufacturers in pharmaceuticals, fine chemicals, or specialty synthesis sometimes rely on it. Transporting such compounds outside national boundaries rarely follows a simple template.
Regulations always trace back to safety, environmental, and sometimes national security concerns. Each country builds categories for chemicals — hazardous, precursor, controlled, dual-use. A compound like 4-chloro-1-methylpiperidinium chloride, if flagged for its synthetic utility or structural similarity to regulated substances, will face closer attention. The European Union, the US, China, and others publish public lists that describe what requires extra paperwork, registration, or even an outright ban.
Sometimes, I’ve seen colleagues spend weeks tracking harmonized system (HS) codes and researching licenses just for one shipment. If the compound can be used as a precursor for drugs, military chemical agents, or explosives, regulators dig even deeper. Shippers and buyers shoulder the risk for misdeclaring a shipment, risking huge financial losses or even criminal charges.
Anyone expecting to move chemicals like this internationally has to gather paperwork far in advance. Safety Data Sheets, certificates of analysis, end use statements, and declarations from buyers are scrutinized by customs officers and carriers. I remember a shipment held in Singapore for almost a month simply because the recipient’s import permit had a typo in the product name — customs refused clearance, and storage costs grew daily.
Shipping companies decide on their own policies too. Couriers such as FedEx and DHL usually ask for proof that the compound fits their acceptable items list and that international transport complies with IATA or IMDG codes. Some won’t touch a package if a chemical appears vaguely suspect, sometimes after broadly interpreting international regulatory updates or news about misused substances.
No ready-made answer exists for all countries, so groundwork pays off. If you plan to ship or receive 4-chloro-1-methylpiperidinium chloride, reach out to local chemical compliance consultants or logistics companies with experience in the field. Verify chemical codes and cross-reference them against restricted and controlled substance lists in both export and import countries. Customs brokers can help navigate rapidly changing rulebooks, and they’ll know what extra documentation fits each port’s requirements.
Supply chain transparency needs ongoing attention. Supply agreements and import declarations should match exactly what's in the box, and buyer checks should reflect the real end-use. Never rely on old approvals: regulations regularly update, and one new listing can reroute entire logistics operations.
Global scientific collaboration depends on sharing compounds for research and discovery. Governments can keep dangerous substances in check without throwing up unnecessary blockades, but this needs open talking between regulators, exporters, and end users. Modernizing customs processes — sharing electronic records and standardizing requirements — could trim down the weeks-long waits and sometimes arbitrary stops.
Shipping 4-chloro-1-methylpiperidinium chloride isn’t straightforward, but responsibility, accurate research, and honest paperwork make it possible for those who genuinely need it for innovation and discovery.

| Names | |
| Preferred IUPAC name | 1-Methyl-4-chloropiperidin-1-ium chloride |
| Other names |
N-Methyl-4-chloropiperidinium chloride 4-Chloro-1-methylpiperidine hydrochloride 1-Methyl-4-chloropiperidinium chloride |
| Pronunciation | /ˈfɔːr klɔːr.oʊ waɪn ˈmɛθ.əl paɪˈpɛr.ɪd.i.əm ˈklɔːr.aɪd/ |
| Identifiers | |
| CAS Number | 15797-57-8 |
| 3D model (JSmol) | `3Dmol.js:JSmol.loadInline('data:chemical/x-pdb;base64,HDRvCg==')` |
| Beilstein Reference | Beilstein Reference: 60562 |
| ChEBI | CHEBI:82778 |
| ChEMBL | CHEMBL2105786 |
| ChemSpider | 19918645 |
| DrugBank | DB11451 |
| ECHA InfoCard | 03a4759c-a145-4d23-88ce-4430ebdc80f0 |
| EC Number | NA |
| Gmelin Reference | 107022 |
| KEGG | C18666 |
| MeSH | D017609 |
| PubChem CID | 126872 |
| RTECS number | GU7176000 |
| UNII | I0Q415AMD5 |
| UN number | UN3241 |
| Properties | |
| Chemical formula | C6H15Cl2N |
| Molar mass | 196.09 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.12 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.9 |
| Acidity (pKa) | pKa = 11.2 |
| Basicity (pKb) | pKb = 4.19 |
| Magnetic susceptibility (χ) | -77×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.518 |
| Dipole moment | Dipole moment: 5.28 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 171.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and serious eye irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P332+P313, P337+P313 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Lethal dose or concentration | LD50 (oral, rat): 570 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 705 mg/kg |
| NIOSH | DH8225000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m3 |
| IDLH (Immediate danger) | NIOSH has not established an IDLH value for 4-Chloro-1-Methylpiperidinium Chloride. |
| Related compounds | |
| Related compounds |
4-Chloropiperidine 1-Methylpiperidine 4-Chloro-1-Methylpiperidine Piperidinium chloride N-Methylpiperidinium chloride |