Looking back, chemists started using Boc protection in the mid-1900s as they pushed deeper into peptide synthesis. The appearance of 4-Boc-piperazine followed naturally as synthetic labs wanted controlled, step-by-step modifications without losing the flexible structure of piperazine. Over the years, the combination of piperazine’s backbone with the Boc group’s manageability gave both academic and industrial teams something new to build on. By this point, a simple amine like piperazine gained a way to block one nitrogen for targeted reactions, which fueled interest everywhere from pharma research to teaching labs. The rise of combinatorial chemistry brought these tools out of the shadows, and now it’s rare to see a molecular library without at least a few Boc-protected piperazines on the roster.
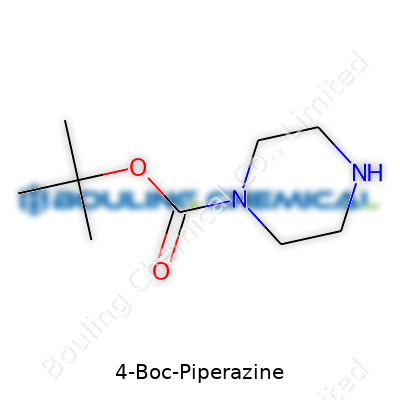
4-Boc-piperazine sits on the shelf as a pale solid or crystalline powder, a clear sign of its stability under standard lab conditions. Suppliers usually offer it in bottles labeled for synthetic intent, highlighting purity above 98%. The molecule wears a tert-butyloxycarbonyl (Boc) group at the nitrogen atom in the four-position of piperazine’s ring, which gives it unique selective reactivity compared to unsubstituted partners. This protective group isn’t just for show — it lets chemists plan multi-step routes with confidence, protecting one part of the molecule while the other stays reactive. I’ve seen it featured in catalogs targeting small-molecule synthesis, med-chem teams, and custom peptide builders, since everyone appreciates predictability in a toolkit.
4-Boc-piperazine carries a molecular formula of C9H18N2O2 and checks in with a molar mass of roughly 186.25 g/mol. Its melting point hovers near 80°C. Solid at room temperature, the powder tends toward white but can yellow slightly if left exposed too long. Most organic solvents dissolve it readily, although chewed-up glassware can be a sign of too much time in basic media. The Boc group behaves as expected, stable in basic conditions but gone quickly in strong acid. You don’t see much volatility with 4-Boc-piperazine, and the compound keeps its integrity refrigerated or desiccated. In solution, it holds up through moderate heating and resists most forms of light-induced breakdown.
Product labeling lists purity — usually HPLC assessment above 98%, as lower grades risk byproduct headaches. Labels also show storage advice, often: “Keep dry, refrigerate, and prevent strong acid contact.” Standard documentation — SDS, CoA, and sometimes NMR or LC/MS — rides along for any regulated sale. A decent supplier includes batch-specific info, so if something goes wrong downstream, you can trace it to the source. Teams in regulated spaces, especially pharma, lean heavily on these technical sheets to pass audits, tie raw ingredients to production trails, and satisfy internal quality checks.
The route to 4-Boc-piperazine starts with piperazine itself. Reaction with di-tert-butyl dicarbonate (Boc2O) under base, like triethylamine, at room temperature leads to selective Boc protection at one nitrogen. Careful stoichiometry and slow addition of reagents prevent twin protection and messy products. Workups go through aqueous extraction, drying, and slow evaporation to avoid premature deprotection. Most teams keep the process scalable, and small-lab syntheses only differ from commercial prep in equipment size. Final purification often uses recrystallization in ether or hexane, delivering a product dry and free-flowing enough for weighing in gloveboxes or at open benches, depending on handleability needs.
Once made, 4-Boc-piperazine swaps out the Boc with little fuss, typically under TFA or strong HCl. That’s just the beginning. The free nitrogen across the ring grins at alkylation, acylation, and sulfonylation reactions, making for rich structure-activity relationship studies in medicinal chemistry. The Boc group holds out through most basic or neutral transformations, meaning you can tack on fluorophores, pegylated groups, or crosslinkers before removing protection for further steps like peptide coupling. In my experience, it’s this reliable switch — protected until you don’t want it — that makes Boc-piperazines valuable for staged synthesis. The molecule sees frequent use in building up heterocycles, fragment-based screening libraries, and as an intermediate on the path to more elaborate drugs.
You might see 4-Boc-piperazine under several handles: N-Boc-piperazine, Piperazine-1-carboxylic acid tert-butyl ester, or even tert-butyl 4-piperazinylcarboxylate. Catalogs sporadically toss out Systematic or IUPAC names, but most lab folks know it simply as 4-Boc-piperazine. Some specialty vendors might bundle minor modifications — deuteration, labeled isotopes — but the basic backbone stays the same. I’ve heard colleagues refer to it by whatever stockroom label fits, so clear alias tracking matters if you want the right bottle for the next step.
4-Boc-piperazine gets the same respect as other synthetic intermediates: gloves on, minimal dust, open bottles in ventilated hoods. The compound doesn’t pose outsized acute toxicity, but skin, eye, and respiratory irritation can show up after poor handling. On larger scales, powder mishandling leads to inhalation trouble. Spill response means sweeping, not vacuuming, as static-charged dust brings exposure risks. Disposal typically follows organic waste practices, and waste storage away from strong acids is wise, since acid-promoted deprotection boots off the Boc, leading to free amines with different hazard profiles. Teams delivering batches for clinical or GMP high-purity work check for residual solvents, unreacted Boc2O, and any unwanted byproducts before blessing the shipment.
Med-chem researchers choose 4-Boc-piperazine for practical reasons. The piperazine core shows up in many drug candidates — antipsychotics, antihistamines, kinase inhibitors, maybe even anti-virals. Accurately protecting one nitrogen opens doors to scaffold hopping, creating analog families in just a few steps. When teams jump into SAR campaigns, Boc-protected intermediates speed up analog prep and keep workflows smooth when timeline crunch hits. In material science, you see it among ligand precursors or in areas needing controlled amine introduction. The biotech sector often grabs it for coupling with peptides, and custom reagent suppliers mix Boc-piperazines into linker toolkits for antibody-drug conjugates or similar payload projects.
R&D pushes boundaries around 4-Boc-piperazine. I’ve seen labs working refreshing new coupling partners, evaluating milder Boc-removal protocols for sensitive compounds, and exploring “green chemistry” approaches to its synthesis. Automated synthesis platforms, like those in DNA-encoded libraries, lean on predictable Boc chemistry to avoid multi-step troubleshooting. The rise of photoredox techniques has led some groups to test non-traditional modifications, hoping to swap functional groups on the backbone or ring without knocking off the Boc unless it’s the last resort. Some analytical chemistry teams use the compound as an anchoring point for developing new separation methods when handling multiply-protected species gets unwieldy.
Toxicology data for 4-Boc-piperazine don’t hit the top-tier journals, but teams tend to extrapolate from both its base structure and related piperazines. Acute exposure ranks low in terms of harm; standard short-term handling brings redness or slight irritation, nothing overtly dangerous under normal lab protocols. Chronic effects have not attracted significant attention due to limited direct use in consumer products, though any intermediate with a reactive amine backbone should be handled sensibly. In risk assessments for pharmaceutical intermediates, the piperazine core always triggers checks for CNS or blood-brain barrier activity, but Boc protection drops bioavailability, lowering worries for those making bulk lots in a closed system. Still, with all synthetic intermediates, unknown contaminants or process residues invite ongoing vigilance.
Chemistry keeps advancing, and 4-Boc-piperazine appears set for as much use as ever. As more blockbuster drugs use the piperazine ring, and as modular synthesis gains traction in drug discovery, this compound won’t slip away from lab shelves. Expect new prepping methods using recyclable reagents, probably less reliance on volatile solvents, and automation that offers tighter control of purity or waste output. As bioconjugation and tagging demands climb higher, 4-Boc-piperazine will anchor more newcomers to amine chemistry, turning challenging syntheses into approachable, reliable routines. It’s an approachable, reliable workhorse, yet it still promises new solutions as synthetic demand rises.
Walk into any modern chemistry lab and one of the easier molecules you’ll find getting used for syntheses is piperazine. Pop a Boc (tert-butoxycarbonyl) group on it, and suddenly you’re looking at 4-Boc-piperazine, a compound that seems, on the surface, pretty straightforward. The real story runs deeper than a simple chemical structure — it’s a tale about practicality, flexibility, and the careful art of protecting functional groups.
Start with piperazine: a six-membered ring, four carbons, and two nitrogens sitting across from each other. Think of it as a close cousin to the more well-known piperidine, just with a pair of nitrogens instead. Chemists took a look at this ring and realized you could attach a group to one of those nitrogens, specifically at the spot chemists call “position 4.” That’s where the Boc group comes in: tert-butoxycarbonyl isn’t fancy for the sake of being fancy — it’s a bulky protective shield. It tacks onto the nitrogen via a straightforward carbamate linkage and takes up so much space that fragile sections of the molecule stay safe during harsh reactions.
Looking at this in shorthand, you’d see: N-tert-butoxycarbonyl piperazine, or more clearly, a piperazine ring with one nitrogen protected by a Boc group, while the other nitrogen stays “free.” Boc’s bulky arms stick out, shielding that part of the molecule much like a goalie guards the net.
Anyone who’s worked in medicinal chemistry knows how valuable a good protecting group can be. The Boc group’s popularity isn’t just luck — it allows for chemical reactions that otherwise could wreck a sensitive amine. Medicinal chemists chase new drugs by piecing together building blocks, and delicate molecules often call for some clever shielding to survive the ride. Strip the Boc at just the right step, and you’ve got a clean amine, ready for the next connection.
The ability to remove the Boc group with simple acid treatment also means you control exactly when the molecule is exposed. There’s no magic — just knowledge of acid-base chemistry and patience. Sometimes, the difference between a failed synthesis and a blockbuster drug rests on one group like this.
Using 4-Boc-piperazine isn’t just about structure, it also brings a few headaches. Boc-protected piperazines can handle a good amount of abuse from heat and moisture, but they don’t like strong acids for too long. You have to plan every step, especially if there are other protecting groups involved. Miss a detail and you might lose your precious amine along with the Boc group.
Purification after the protection or deprotection step can cause some frustration — sticky residues, stubborn byproducts, and the constant hassle of making sure all the protecting group is gone. I’ve spent hours running columns just to chase out every last bit of Boc.
With industry always pushing for cleaner, faster routes, chemists keep searching for milder deprotection conditions and more sustainable methods. Automation helps by taking some drudgery out of repetitive procedures. Alternative protecting groups are popping up, but Boc holds on because chemists trust it. Cleaner chemistry means more than just structure; it’s about making every step count, saving time, and increasing the chance that a good idea turns into a working drug.
4-Boc-Piperazine shows up a lot on the benches of research chemists working on new medicines. Think of it as a helpful building block, especially when shaping molecules that might someday become treatments for cancer, infections, or mental health issues. It’s like having a favorite wrench in a toolbox—reliable, flexible, and always needed for a range of jobs. Looking at the surge in drug design over the last couple decades, you’ll find 4-Boc-Piperazine in the recipes for all sorts of experimental compounds.
Protecting groups sound technical, but they play a simple role: they let scientists put together larger, more complicated molecules without the whole works going haywire. The “Boc” in 4-Boc-Piperazine helps shield part of the molecule during chemical reactions. So, if you want to connect the piperazine part to something else without unwanted side effects, the Boc group gives you a bit more control. This has always struck me as a good reminder of how science sometimes moves ahead not with major discoveries, but with steady, practical tweaks like this—saving time, money, and frustration.
Scan the research coming out of biotech companies, and you’ll start to notice how 4-Boc-Piperazine slips into projects looking for new anticancer agents or drugs aimed at the brain. Researchers link this molecule to other parts, trying to find combinations that target tumors or help the nervous system function better. It’s not glamorous, but these building blocks pile up progress, one experiment at a time. There’s been a noticeable bump in published results that mention 4-Boc-Piperazine, a sign that researchers trust it when crafting new compounds.
Screening libraries, which sound like medical file cabinets, really just mean batches of chemicals that researchers test against a disease target. 4-Boc-Piperazine fits snugly into these efforts. Teams blend it with other chemicals to whip up hundreds or thousands of slightly different molecules. This scattershot approach sometimes feels tedious, but it leads to surprises—a handful of those molecules get lucky and hit the bullseye, paving the way for the next round of medical breakthroughs.
Safe handling takes on a bigger role in busy labs. 4-Boc-Piperazine’s stability makes it a favorite—less fuss, less risk of unwanted reactions, less headache during storage and shipping. Having worked with more hazardous chemicals myself, I know how much it matters when something just works, with no drama and no worrying about shelf-life or breakdown. These nuts-and-bolts improvements add up to fewer lab accidents and more reliable results.
Maybe the last word belongs with the way 4-Boc-Piperazine brings people together. It’s used by teams around the world, from university departments to startup companies, and it helps everyone speak the same language in medicinal chemistry. Consistent reagents mean scientists can compare data, share findings, and build on each other’s work with more confidence.
As demand grows for faster drug development and greener chemistry, suppliers and scientists keep tinkering with how 4-Boc-Piperazine is made and used. There’s space here for smarter manufacturing processes, less chemical waste, and wider access for researchers in smaller labs. All this makes the story of 4-Boc-Piperazine a reminder that scientific progress often relies on the quiet reliability of its everyday tools.
Some chemicals get a lot of attention for their purity requirements, and 4-Boc-piperazine stands among them. This molecule finds work across pharmaceutical and research fields, seeing heavy use in medicinal chemistry for its ability to build more complex compounds. Most suppliers advertise 4-Boc-piperazine with claimed purities—usually above 98%, with premium suppliers even listing ≥99%. There’s an expectation here: that the powder or crystals in that lab bottle reflect the high numbers on the label.
A couple years back, I watched a colleague frustrated by what seemed like a simple task—synthesizing a promising new compound. He blamed a sluggish reaction, but it didn't take long to see minor impurities in his Boc-protected piperazine. Sure, the product label promised 99%, but a second NMR told a different story: moisture and a load of residual solvents. On paper, the numbers matched; in practice, sideline contaminants affected reactivity.
Anyone investing in organic synthesis work quickly sees the cost of ignoring purity. Impurities play saboteur, joining reactions that need only one player. Even a single percent of unknowns changes outcomes—fouling chromatography, affecting crystallization, or forcing unnecessary purification steps down the line.
Take the U.S. Pharmacopeia’s standards: drug intermediates rarely dip below 98% actual purity because nobody wants to gamble million-dollar processes on off-spec chemicals—especially in regulated environments. Research-grade material, supplied for academic labs, may slide by with 97%–98%. For those scaling things up—pilot plants, pharma manufacturing—nobody bats an eye until the certificate of analysis shows trace-level reporting. Technicians read every specification, searching for water content, residual solvents, or heavy metal traces that quietly complicate downstream processing.
Some might think quality checks for 4-Boc-piperazine offer peace of mind. In reality, purity reporting often boils down to HPLC or GC analysis, and those methods do their job—up to a point. Instrument sensitivity and reference standards really set the ceiling. Even for bottles labeled as 99%, findings can shift between different suppliers or batches. Small shops sometimes cut corners, knowing academic labs rarely double-check. Larger brands guard their reputation, but nobody escapes the risk of a bad lot.
Relying on paper guarantees gets risky in research. I’ve seen teams order a couple of grams from three vendors, test each with TLC and NMR, and bulk-order only after confirming the numbers match the claimed purity. This kind of due diligence saves a ton of troubleshooting headaches.
For larger operations, material enters through quarantine, backs up with a certificate of analysis, and goes through extra analytic checks in-house. Many companies invest in tighter quality audits and regular third-party testing, not just for legal reasons but to avoid wasted weeks chasing down a rogue impurity.
Smaller players—especially academic labs—don’t always have the budget or staff for full workups. A practical tip: keep a small reserve of previously trusted 4-Boc-piperazine, run side-by-side tests with new batches, and document differences before any big synthesis.
Higher purity doesn’t always bring huge expense, but the difference adds up in large-scale work. Cleaning up after a botched reaction often runs pricier than the few extra dollars per gram for a more reliable source. The best path: weigh cost, supplier track record, and in-house testing capabilities. There’s no substitute for knowing exactly what’s going into the reaction flask. This careful approach keeps projects on track, budgets healthy, and results defensible.
Anyone who’s worked in a lab for long enough has a story about an experiment gone sideways because of a chemical left out on a bench, or a bottle forgotten on a windowsill. It might seem overblown, but those moments can ruin months of work, or worse, create a safety headache. 4-Boc-Piperazine, for all its practical uses as a building block in pharma labs and chemical synthesis, isn’t any different. Safe storage matters as much as skill at the bench.
I’ve seen what humidity does to glassware, and I’ve pulled sticky, degraded powders out of containers with faded labels before. Moisture and heat don’t play nicely with chemicals like 4-Boc-Piperazine. Exposing it to the open air cuts shelf life and reliability down to size in a hurry. Left sitting in sunlight, its quality can drift, sometimes in ways not obvious until a TLC plate or NMR spectrum throws a curveball.
Basic rule I stick to: keep reagents in tightly sealed bottles, with as little air inside as practical. Glass with screw-top lids and PTFE liners rarely let me down. Lab supply catalogs know this, so most containers arrive ready for the job. A solid, climate-controlled stockroom should set the pace—room temperature works, but swingy conditions or extra heat set up trouble. I once watched a summer AC breakdown wipe out a week’s worth of product because nobody moved the jars into backup storage.
Many people assume routine chemicals aren’t risky. That attitude sneaks up the food chain, especially with less dramatic-looking powders. 4-Boc-Piperazine has decent stability, but its safety data sheet still calls for gloves and eye protection while handling—powdered organics have a tendency to scatter and coat exposed skin, and who wants to roll the dice on dermal exposure over a routine batch?
Fume hoods aren’t just for the big hitters—storing spare jars or transferring quantities works best with good ventilation, even for “just a standard piperazine.” Ask any experienced chemist about that day they got lazy and ended up sneezing or with irritated hands from “just a little bit” of whatever they brushed up against.
One thing that sticks out from my grad school days is a line of faded bottles on a bottom shelf with cryptic abbreviations and almost mythical origins. It’s an easy trap—label wear wipes out any hope of tracking what’s inside, and unmarked containers invite sloppy work. Every bottle needs a legible label with name, concentration, prep or purchase date, and who opened it last. Adding barcodes or digital inventory helps in larger outfits, but even simple pen-on-tape gets the job done.
Rotation avoids a backup of expired or untrustworthy material. Chemicals don’t always send out clear warnings they’ve gone off, and purity hits can derail tight research timelines. Pull the oldest off the shelf first, and toss any jar past its best-by mark or showing strange color, odor, or texture.
Safe storage happens through habit, not bureaucracy. A small shelf, regular checks, clear labels, and keeping chemical containers out of sunlight and in dry spots make a difference. For 4-Boc-Piperazine, that’s the recipe I trust, and it’s rescued more than one experiment from an unscheduled repeat. Buy in sizes right for your workload, keep a single working jar on the bench, and stash extras sealed away where light and humidity can’t get at them. The simplest routines pay off the most—in science, that's a lesson that sticks.
In the lab, 4-Boc-Piperazine shows up as an off-white solid. It’s a useful tool for chemists, especially those working with drug synthesis or specialized chemical processes. Folks with a bottle of this chemical on the bench should see it for what it is: a compound offering plenty of potential, but zero forgiveness for carelessness. Anyone who’s put on nitrile gloves and weighed out even a gram knows these reagents require steady nerves and simple respect.
The first thing that jumps out, at least to anyone who has spent time in a research building, is how basic rules save skin — and sometimes eyesight. Nitrile gloves, safety goggles, and a sturdy lab coat may seem like window dressing to the uninitiated. Spend ten minutes in a lab and you’ll never forget splash hazards. 4-Boc-Piperazine won’t eat through gloves in seconds, but skin contact isn’t something you want. Not every chemical will burn fast, but chronic exposure creeps up if you’re careless day after day.
Some folks think the fume hood is just for the stinkiest stuff. In practice, the hood’s glass shield and constant airflow form a protective bubble from dust, splashes, and accidental inhalation. Even though 4-Boc-Piperazine isn’t as volatile as some, any fine powder can float up and make a mess of your sinuses.
A common trap: treating every white powder as harmless. Years in the lab drilled into me the habit of checking the SDS (safety data sheet) before opening any new bottle. For 4-Boc-Piperazine, storage in a tightly sealed container, away from strong acids or oxidizers, matters more than you’d expect. Moisture in the air can wreck a batch, and reactive chemicals in the same cabinet may spark more trouble if spilled together.
Weighing small amounts on clean, disposable weighing paper keeps surfaces from becoming invisible hazards. If some lands on the bench, it’s a race to wipe it up with damp paper towels. Dry brushing kicks up dust. One or two experiences cleaning up spills with a dry cloth will send anyone searching for a better method.
Disposal trips up a surprising number of newcomers. Pouring leftovers down the sink or tossing contaminated gloves in the usual trash sounds easy, but it’s a shortcut with real risks. Most labs keep a dedicated container for organic waste and another for solid trash like pipette tips and gloves. Labeling those containers clearly stops confusion, especially late at night when fatigue muddles judgment.
Old-timers know that having access to an eyewash station and spill kit nearby gives peace of mind. No one wants to use them, but accidents don’t send appointments first.
Safety meetings can sound preachy, but the best ones share stories, not just rules. Some of the closest calls come from ignoring small steps: skipping goggles just that one time or trusting memory instead of checking labels. Bringing new people into the fold means explaining not just the how, but the why of each safety measure. Real safety comes from repetition, habit, and encouraging everyone in the room to speak up if they see something off.
Chemical work always brings a measure of risk. The people who leave at the end of the day with all ten fingers and clear lungs usually have the sharpest attention to the boring details. That’s where real safety lives.
| Names | |
| Preferred IUPAC name | tert-butyl 4-piperazinecarboxylate |
| Other names |
1-Boc-piperazine tert-Butyl 4-piperazinecarboxylate N-Boc-piperazine 4-(tert-Butoxycarbonyl)piperazine |
| Pronunciation | /ˈfɔːr bɒk paɪˈpɛrəziːn/ |
| Identifiers | |
| CAS Number | 57260-71-6 |
| 3D model (JSmol) | `3D model (JSmol)` string for **4-Boc-Piperazine**: ``` CC(C)(C)OC(=O)N1CCN(CC1) ``` |
| Beilstein Reference | 1361008 |
| ChEBI | CHEBI:131571 |
| ChEMBL | CHEMBL418561 |
| ChemSpider | 81661 |
| DrugBank | DB07761 |
| ECHA InfoCard | ECHA InfoCard: 100.131.487 |
| Gmelin Reference | 2892366 |
| KEGG | C11970 |
| MeSH | D010999 |
| PubChem CID | 104233 |
| RTECS number | TD1040000 |
| UNII | G6RNM2W3C9 |
| UN number | UN3272 |
| Properties | |
| Chemical formula | C9H18N2O2 |
| Molar mass | 286.38 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.09 g/cm3 |
| Solubility in water | slightly soluble |
| log P | -0.38 |
| Acidity (pKa) | pKa = 9.8 |
| Basicity (pKb) | pKb = 4.27 |
| Refractive index (nD) | 1.488 |
| Dipole moment | 3.59 D |
| Hazards | |
| Main hazards | Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P270, P280, P301+P312, P330, P501 |
| Flash point | 105 °C |
| PEL (Permissible) | No PEL established. |
| REL (Recommended) | 50 mg |
| IDLH (Immediate danger) | NIOSH does not list an IDLH value for 4-Boc-Piperazine. |
| Related compounds | |
| Related compounds |
1-Boc-piperazine N-Boc-piperazine piperazine 4-methylpiperazine 4-phenylpiperazine |