Interest in 4-aminomorpholine goes back to the postwar period, when industrial chemists looked for building blocks that could power the next round of synthetic drugs and polymer materials. Unearthing the simple six-membered morpholine ring and swapping out a hydrogen for an amino group kickstarted its story. Laboratories in Europe and the United States dove into heterocycles, exploring their reactivity and pharmacological profile. This one, with its unique pattern of nitrogen and oxygen, soon attracted the attention of researchers piecing together new avenues in medicinal and industrial chemistry, especially at a time when new drugs and pesticide ingredients were in fierce demand. The pathway from simple amines and ethylene oxide found its way into textbooks and patents, helping to define synthetic routes for decades.
4-Aminomorpholine stands out as a versatile intermediate. Chemists value it as a starting material for pharmaceuticals, agricultural chemicals, and specialty polymers. It carries the morpholine structure, which offers a balance between water solubility and basicity. This means it can slot neatly into multistep syntheses for more complex targets. Today, you’ll find it available from specialty suppliers in both bulk and research grades. Custom formulations do exist, but the core product stays the same: a light, crystalline powder with a faint amine odor, kept in tightly sealed containers to avoid moisture absorption and degradation.
If you pour out 4-aminomorpholine on the bench, you’re dealing with a solid that melts around 129°C and boils just above 250°C. Its water solubility matches that of many simple amines, so you won't fight with dissolving it for most reactions. The amine and ether groups both draw in water molecules, which means you need to pay attention to humidity in your lab. The ring offers remarkable chemical stability under most conditions, holding up well against hydrolysis. The basic nitrogen offers a point of attack for acids, while the secondary amine grabs electrophiles during functionalization. Measuring pH solutions or setting up a recrystallization will feel familiar if you have experience with other small organic bases.
4-Aminomorpholine packaging typically remains straightforward: bottles or drums labeled with CAS number 3524-74-1, chemical formula C4H10N2O, and a lot number for traceability. Purity hovers between 97% and 99%, with moisture content noted to help users keep track of storage quality. As with most research chemicals, labels warn of health hazards and recommend basic personal protective equipment. Some suppliers add recommended storage temperature and shelf life, but the chemistry doesn't demand elaborate environmental controls—just dry, cool storage away from incompatible acids.
There are a couple of widely used synthetic routes for producing 4-aminomorpholine. One reliable approach comes from reacting morpholine with ammonia derivatives under pressure, sometimes using catalysts to boost yields. Another method starts with diethanolamine, which offers hydroxyl groups ready for nucleophilic substitution; introducing an amination reagent converts the alcohols into the sought-after amine. Some labs stick with classic Gabriel synthesis variations, using phthalimide intermediates, but most industrial-scale plants favor high-yield, direct amination to cut out the extra steps. Choosing a method depends on access to starting materials, waste disposal regulations, and cost efficiency—concerns that echo across the fine chemical industry.
The simple structure of 4-aminomorpholine actually opens the door for lots of transformations. Acylation converts it into ureas and amides, making it a handy intermediate for pharmaceuticals. The amino group reacts smoothly with aldehydes in reductive amination, providing access to more complex heterocycles. The oxygen atom in the ring can, under strong acidic or oxidative conditions, participate in ring opening or rearrangement—though most chemists stick to milder conditions, preserving the morpholine framework. Metal-catalyzed couplings, like Suzuki or Buchwald-Hartwig reactions, use the amine as an entry point for introducing aromatic or alkyl chains. Each reaction expands its reach as a synthetic tool, especially for those piecing together complicated drug scaffolds or high-performance polymer additives.
Chemists don’t always agree on naming, especially with something as basic as this. You’ll see 4-aminomorpholine also called morpholin-4-amine, N-amino morpholine, and the systematic 4-morpholylamine. Some suppliers just stick with a trade name or simple abbreviation like 4-AM or AMF. Checking the CAS number usually clears up confusion between isomers and analogs, since similar-sounding names pop up across catalogs.
Handling 4-aminomorpholine is a bit like working with other small amine compounds: gloves, goggles, and lab coats are your best defense. Accidental contact can cause irritation, so fume hoods keep vapors in check during reactions or weighing out powder. The literature doesn’t show a history of fires or explosions under standard conditions, but it makes sense to store it away from oxidizers and acids to avoid unintended reactions. Facilities dealing with larger quantities post clear signage and provide spill kits, since basic amines can grab onto acids in plumbing or ventilation systems, corroding metals or plastics over time. Standard first aid—flushing eyes, washing skin, getting fresh air—handles most minor accidents. Safety Data Sheets back up common-sense procedures.
As a workhorse intermediate, 4-aminomorpholine shows up in pharmaceutical labs, agricultural chemistry, and material science. It gets routed into cough suppressants, anti-inflammatory drugs, and antifungal agents—a testament to its utility in medicinal chemistry. Polymer chemists value amine-substituted heterocycles as building blocks for specialty coatings and ion-exchange resins. Agrochemical labs find it useful for synthesizing active ingredients that end up in crop protection products. The diversity of applications comes as no surprise to anyone familiar with nitrogen-containing rings, which offer a platform for tuning solubility, reactivity, and biological activity.
R&D labs haven’t exhausted the options for 4-aminomorpholine. Work continues on using it as a precursor for kinase inhibitors and other enzyme-targeted medicines. Screening for new antifungal and antibacterial properties occasionally uncovers surprises based on small modifications of the morpholine template. Material scientists look into new polymers with improved electrical and mechanical properties, using the amine’s reactivity to anchor functional groups. Advances in green chemistry even focus on making its production more efficient, with fewer hazardous byproducts and lower energy demands, reflecting a wider trend toward sustainability across the industry.
Concerns about toxicity reflect the broader pattern with small amine-containing chemicals. Acute exposure in animal models produces mild to moderate irritation at high doses; the low vapor pressure keeps inhalation risks manageable in properly ventilated spaces. Long-term studies haven’t identified a significant cancer risk, though the compound can cause sensitization on repeated skin contact. Environmental studies show that rapid biodegradation limits accumulation, but wastewater with high concentrations should undergo chemical treatment before disposal. Careful labeling and regular training reduce accidental exposures, keeping risks manageable. Regulatory agencies track occupational exposure, but the absence of major incidents speaks to effective controls in most facilities.
As the chemical industry focuses on more targeted drugs and advanced materials, 4-aminomorpholine still shows strong potential. Pharmaceutical companies push for more selective, potent compounds using the morpholine scaffold as a launch pad for discovery. Green chemistry keeps driving process improvements, favoring reactions that generate less waste or run at lower temperatures, which could trim costs even as safety improves. Digital modeling and AI-guided synthesis might unlock new derivatives for diagnostics, therapy, or advanced materials, offering new directions for a molecule that started out as just another postwar laboratory curiosity. Cheaper, cleaner routes don’t just help big industry—they make it possible for research teams everywhere to keep innovating with confidence, knowing that an old favorite still has fresh surprises up its sleeve.
4-Aminomorpholine doesn’t end up on billboards or headlines, but it does some heavy lifting in chemistry labs and manufacturing plants. At heart, this stuff serves as a handy building block for all sorts of chemicals. If you’ve ever tried your hand at making something useful from scratch—think baking bread or putting together furniture—it’s clear that having the right ingredients matters. 4-Aminomorpholine works like flour in chemistry; you see it at the start so scientists can make bigger, more complex things.
People often overlook the early steps in drug development. Chemists lean on 4-Aminomorpholine to piece together key components in medicines. It helps build molecules that block or activate processes in the body. Those processes show up in treatments for certain infections and even cancer therapies. Cancer drugs, for instance, depend on specific molecular shapes—4-Aminomorpholine helps shape those pieces, carving out a molecular backbone that other parts stick to. In practice, this means researchers have another tool for solving puzzles in drug design.
Pharmaceuticals are only one patch of the landscape. 4-Aminomorpholine steps up in creating specialty chemicals and polymers, especially where toughness or chemical resistance is a must. It helps fashion coatings, adhesives, and resins. Manufacturers can tweak properties—maybe they want something more flexible, or something tougher against heat. That sort of control is a big deal in aerospace, electronics, and vehicles. In my experience, working with specialty coatings means keeping an eye on the starting chemicals, and this compound crops up more than you’d expect, because it’s good at holding things together in tough conditions.
Researchers treat 4-Aminomorpholine like a helpful wrench in the chemical toolbox. Try making new molecules without these helpers—you’d spend all day on tasks that should take hours. It often appears as an intermediate, one stop in a much longer chain of reactions. Academic labs need compounds like this to test out new reactions, to play with molecular shapes, or to patch together prototypes for commercial products.
Chemicals like 4-Aminomorpholine call for a serious respect. Its reactivity means keeping goggles on and following protocols. Companies doing large-scale production need to mind environmental rules, since leaking chemicals can contaminate soil and water. Tight oversight on transport and storage lessens the risk, yet I’ve seen smaller labs cut corners, thinking their batch is too small for concern—usually, that kind of thinking invites problems. Stricter training and regular inspections solve small problems before they grow teeth.
Demand sits on how quickly science moves in pharmaceuticals and materials science. As new drugs target tougher diseases and industries ask for smarter, cleaner materials, the calls for chemicals like this one keep coming. It isn’t the name printed on the box, but it sure shapes what ends up inside.
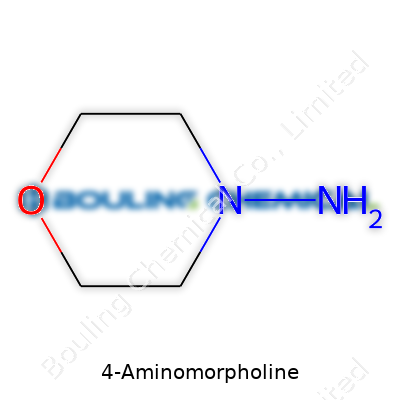
Walking through a lab, the name 4-Aminomorpholine barely raises an eyebrow among most researchers. Its structure, though, can play a much bigger role than its inconspicuous presence suggests. The compound falls in the morpholine family—a class that comes up often in pharmaceutical and chemical circles. Its chemical formula is C4H10N2O. The structure brings together a six-membered ring, combining four carbon atoms, one oxygen atom, and a nitrogen atom that makes up morpholine itself. Then, toss an amino group (-NH2) at the fourth carbon, and you get 4-aminomorpholine.
Think about this molecule as a puzzle. The ring provides stability and flexibility, making it useful as a building block. Scientists like it for this reason alone. Add the amino group, and it becomes even more interesting. That small group boosts its ability to make hydrogen bonds. In practice, this opens up doors for reactions and can drive innovation in medical research. It reminds me of simple experiments in college chemistry labs: adding a small group to a molecule always seemed to change the game completely.
I’ve seen plenty of experiments where a tweak in structure leads to big changes in how a substance behaves. That’s the reality here too. For 4-aminomorpholine, researchers have found that its structure offers a neat starting point for synthesizing more complex chemicals. Some companies introduce it as a stepping stone when crafting drugs — a backbone for groundbreaking treatments.
So what does the structure look like? Imagine a hexagon. One corner is oxygen, one is nitrogen, and the other four are carbons. At carbon number four, the amino group hangs off the ring. Straightforward, but this setup holds real value. I remember seeing something similar on paper for the first time and realizing how even slight changes could give people better drugs or more efficient manufacturing pathways.
Pharmaceutical developers value this ring system. It lets chemists fine-tune molecules for solubility, binding affinity, and many other properties crucial for drug development. That’s not just talk. Morpholine derivatives already appear in antifungal medications and chemical inhibitors. With the extra amino group stuck on, researchers expect even more tailored applications, whether that be anti-cancer compounds or more selective enzyme blockers.
There's always a flipside. Blending new chemical structures into proven processes brings uncertainty. Safety data can lag when compounds stray from the mainstream, meaning chemists must work through a tangle of regulations and risk assessments. In my experience, the gap between a promising structure and an actual product often comes down to time and money spent in testing. Mistakes have real consequences; things that look harmless on paper might surprise in real-world settings.
A smoother path would mean sharing more structural data early and getting regulatory bodies on board right from the research phase. Open databases and better collaboration between industry and academia could help. Training new chemists to look beyond the surface of molecular diagrams—learning what those little changes actually mean for activity and safety—might help too. Connecting a chemical’s structure with its bigger-picture impact won’t just help researchers, it gives everyone else a stake in future discoveries.
4-Aminomorpholine doesn’t have the friendliest reputation in the lab or storage room. I remember fresh out of college, opening a cabinet and seeing flammable warning labels stacked on certain bottles. The one that made me extra cautious was 4-Aminomorpholine. This chemical carries a punch due to its mild corrosiveness and a tendency to react if left in the wrong place or exposed without care. One spill on a benchtop taught me quickly—never be careless with stuff like this.
Safe storage means picking a spot that’s both cool and dry. Moisture causes trouble for many chemicals, and 4-Aminomorpholine is no exception. You’ll want air-tight containers, sealed tightly after every use. I always triple-checked lids before finishing up for the day, and it saved me more than once from a clean-up job I’d rather avoid. Glass containers or high-density polyethylene bottles keep it secure. Schools and research labs sometimes skimp, but a little investment here goes a long way.
Sunlight isn’t a friend here either. Direct exposure over time can weaken containers and degrade chemicals. Shelving with opaque doors or a dedicated chemical cabinet blocks both light and accidental access by someone not wearing the right gear.
Nothing beats a well-labeled bottle. I remember a professor telling me that plain labels invite mistakes. 4-Aminomorpholine deserves clear notes about contents, hazard class, and the date it was first opened. Keeping an up-to-date chemical inventory helps people spot old or expired material before it becomes a ticking clock in the storeroom. Even a sharp student can mix something up if the label is faded or missing.
I’ll always picture those goggles fogging up while I wrestled a tight cap off a bottle, but you can’t skip protective equipment. Nitrile gloves, full-length coats, and goggles are basic, and sometimes a face shield offers peace of mind if you’re handling a gallon instead of a beaker. Proper ventilation helps here—a fume hood isn't just a luxury—breathing in vapors over time can cause a slew of health symptoms. Folks working in rooms with no airflow risk headaches, eye irritation, or worse.
Anyone storing or handling this chemical should know their spill response drill—no pretending it “won’t happen to me.” A minor leak or small splash on a bench can corrode surfaces and cause skin burns. Absorbent pads and neutralizers belong in arm’s reach. I once saw a student react quickly and trap a spill with a basic spill kit, which kept the clean-up easy and protected everyone nearby.
Disposal slips sometimes in a busy lab. Sending extra or old chemicals down the drain risks environmental harm. Most cities collect hazardous chemical waste every few months—using this route stops pollution and keeps water sources safe for everyone. Having a solid plan and using labeled waste containers avoids confusion and accidents later.
A culture of training and double checks cuts down on accidents. At my first lab job, a short weekly review of chemical storage policies kept people sharp, and a rotating checklist meant nobody overlooked the basics. As rules get ignored, problems creep in. Training gives everyone the confidence to call out trouble before it turns into a reportable incident.
4-Aminomorpholine rarely turns up in everyday conversation, but it matters to chemists, manufacturers, and anyone who works with specialty chemicals. Its main utility shows up in research or production of drugs or specialty polymers. Whenever someone encounters a substance like this, there’s a simple reality to face: chemicals with complex structures often carry risks. With 4-Aminomorpholine, these concerns start early, before anyone ever opens a bottle in the lab.
Plenty of professionals have learned the hard way that skin and eyes don’t react kindly to basic amines. A quick splash, a bit on the glove, and you feel that telltale burning. 4-Aminomorpholine generally falls into the same category. People working with it would want to keep it off their skin and far from the eyes—protective gloves and goggles aren’t just for show here. Reports and safety data point to moderate skin and serious eye irritation if it slips past your safety barrier. That’s something I’ve seen with other chemicals in the same family, and I've never forgotten how fast that pain starts.
Inhalation stands out because the respiratory system offers no forgiveness. During years in the lab, I learned to respect amines for their sharp, pungent smell—not just because it’s unpleasant, but because breathing in small molecules like these can irritate airways and lungs quickly. Exposure limits remain uncertain, which means one whiff might not seem like much, but daily or even regular exposure piles up risk. Labs and factories owe it to their workers to install solid ventilation, not just an open window. Respirators should be on hand if there’s any doubt.
Swallowing chemicals might sound unlikely, but cross-contamination happens. Someone snacks in a lab, or wipes their mouth with a contaminated glove—these situations seem harmless until symptoms appear. Ingestion of 4-Aminomorpholine likely causes nausea, discomfort, and possibly toxicity, though real data remains thin. The lack of robust studies on long-term or chronic toxicity doesn’t give anyone license to take chances—so it’s best to operate on the safe side, using chemical hygiene practices consistently.
Like many organic compounds, 4-Aminomorpholine poses a fire risk, especially in higher temperatures or near open flames. Many folks in chemical production have tales of unexpected ignition—not always dramatic, but enough to remind that fire extinguishers and spill kits have a permanent place in this kind of environment. Spills create immediate risks, both from fumes and direct contact, so quick cleanup and proper disposal matter as much as any high-tech instrument in the room.
Key safety steps aren’t glamorous, but they work. Good gloves, goggles, and clear labeling keep accidents in check. Labs and plants need well-trained staff, backed by up-to-date safety data sheets and regular drills. Open communication between workers, clear labeling, and a culture that values asking questions all help everyone go home safe. Safety showers and eyewash stations should never gather dust. Lastly, waste needs careful management—no dumping, no shortcuts, and respect for both staff and the environment.
People who use 4-Aminomorpholine, or who work around it, benefit from the lessons others have paid for with pain or near-misses. Training, attention to detail, and humility save more people than bravado. It only takes one mistake to understand why safety rules exist. Substituting less hazardous alternatives makes sense if the chemistry allows, and reviewing every protocol for weak points keeps everyone alert. People—not processes—make safety real, every day.
In labs and chemical plants, 4-Aminomorpholine looks unassuming. Most people never see it in daily life, yet it plays a role in quietly shaping tech, industry, and even our health. Sometimes the value of a chemical shows up in ways you wouldn’t expect. 4-Aminomorpholine doesn’t make headlines, but its behind-the-scenes impact stays steady.
For anyone who’s mixed compounds or dug into chemical synthesis, 4-Aminomorpholine shows up as that useful intermediate with more adaptability than its simple structure suggests. In my days doing research, it served as a backbone for more complicated molecules. Pharma folks use it to put together new antibiotics, cancer drugs, and test compounds. I remember one early project where it was the key to avoiding some nasty, poorly soluble impurities. It saved us hours of troubleshooting, a detail that matters when you’re racing a deadline.
Drug developers like molecules that are both reactive and easy to tweak, which explains the steady demand here. Chemists bond 4-Aminomorpholine with other building blocks, chasing after the next effective antiviral or anti-inflammatory agent. The morpholine ring lends stability. That amino group opens the door for chain reactions. You see it appear in patent filings worldwide, every time a team thinks they’ve found a treatment that could help people in pain or fighting infection.
Medicines aren’t the only outcome. Some factories add this compound to formulations for making specialty resins, coatings, or chain extenders for plastics. Performance plastics show up everywhere, from car dashboards to electrical components. Small tweaks in the chemical backbone determine whether these materials can hold up under heat or stress. I once worked on a project testing polymer blends where just a sprinkle of this compound shifted a product’s melting point—big deal if you want components that last through summer heat.
In other cases, 4-Aminomorpholine shows up in research labs studying how molecules behave under pressure or extreme environments. Analytical chemists favor predictable, clean-reacting building blocks. Whether tweaking high-strength epoxies or chasing after better adhesives, chemists keep coming back to simple amines like this one.
Every chemical poses a double-edged sword: unlock innovation, keep safety on track. Workers handling 4-Aminomorpholine wear gloves and eye protection since it can irritate skin and eyes. Some groups push for greener, safer alternatives or stronger protocols. Research always runs into these questions: "How much risk is worth a payoff?" "Can we redesign molecules to get results without so many hazards?"
Some companies have already swapped to less toxic amines, and green chemistry pushes this trend further. In my experience, the best results come from teams that share safety data and rethink steps before problems show up. Substituting raw materials or using closed systems won’t fix every hazard, but they buy time until someone invents a better answer. That’s what real progress looks like—steady work, careful decisions, sharing what works and what burns out.
| Names | |
| Preferred IUPAC name | 4-Aminooxazinane |
| Other names |
Morpholin-4-amine 4-Amino-morpholine N-Aminomorpholine |
| Pronunciation | /ˈfɔːr əˈmiːnoʊ ˈmɔːrfəˌliːn/ |
| Identifiers | |
| CAS Number | 4394-85-8 |
| 3D model (JSmol) | `3D model (JSmol)` string for **4-Aminomorpholine**: ``` C1COCN(C1)N ``` |
| Beilstein Reference | 1360735 |
| ChEBI | CHEBI:139644 |
| ChEMBL | CHEMBL48837 |
| ChemSpider | 21106382 |
| DrugBank | DB08319 |
| ECHA InfoCard | 03e4d1d6-0e81-45b4-afe7-8bbee3fb50b0 |
| EC Number | 214-225-4 |
| Gmelin Reference | 8035 |
| KEGG | C209384 |
| MeSH | D021888 |
| PubChem CID | 13993 |
| RTECS number | QE8400000 |
| UNII | N3T2R2S3KW |
| UN number | UN2810 |
| CompTox Dashboard (EPA) | urn:cpdat/compound/DTXSID0030622 |
| Properties | |
| Chemical formula | C4H10N2O |
| Molar mass | 104.14 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Amine-like |
| Density | 1.102 g/cm3 |
| Solubility in water | Soluble |
| log P | -1.22 |
| Vapor pressure | 0.0296 mmHg (25°C) |
| Acidity (pKa) | 9.6 |
| Basicity (pKb) | 4.86 |
| Magnetic susceptibility (χ) | -48.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.530 |
| Viscosity | 1.36 cP (at 20 °C) |
| Dipole moment | 2.73 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 322.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -125.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2457 kJ/mol |
| Pharmacology | |
| ATC code | N01AX14 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06, GHS08 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. H314: Causes severe skin burns and eye damage. |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | 86°C |
| Autoignition temperature | 235 °C |
| Explosive limits | Explosive limits: 6–14% |
| Lethal dose or concentration | LD50 (oral, rat): 320 mg/kg |
| LD50 (median dose) | LD50 (median dose): 940 mg/kg (oral, rat) |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 4-Aminomorpholine: Not established |
| REL (Recommended) | 5 g |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Morpholine 2-Methylmorpholine 4-Methylmorpholine N-Phenylmorpholine 4-Aminopiperidine |