In the early 20th century, organic chemists pushed the boundaries of synthetic chemistry, searching for novel morpholine derivatives that could meet the growing needs of industries and academic research. 4-Acetylmorpholine started as one such offshoot, first documented in European research circles, drawing curiosity due to its stability and unique reactivity. Researchers had to tinker with morpholine chemistry, introducing acetyl groups in unique positions to tweak both physical and chemical traits. Over the decades, the compound morphed from an obscure research chemical to something with real utility in modern laboratories, chemical supply houses, and specialty synthesis processes.
4-Acetylmorpholine stands out due to its simple structure yet diverse reactivity profile. At its core, the molecule features a morpholine ring substituted at the 4-position with an acetyl group. Easy to handle at room temperature and often available as a crystalline solid or oily liquid, this compound forms one of many bridge molecules connecting classic heterocyclic chemistry with targeted, value-driven applications. Laboratories and manufacturers have kept it in rotation for both trial syntheses and scale-up projects due to its practical blend of stability and reactivity.
On the bench, 4-Acetylmorpholine typically appears as a colorless to pale yellow liquid or sometimes a low-melting solid, depending on sample purity and ambient conditions. The chemical formula is C6H11NO2, with a molecular weight of 129.16 g/mol. Most samples show a melting point just below room temperature and a boiling point north of 250 °C. The acetyl substituent noticeably affects the electron density around the ring nitrogen, softening basicity and altering solubility characteristics. This morpholine derivative dissolves well in common organic solvents like ethanol, acetone, and ether, which helps chemists working on derivatization and purification. Its spectral fingerprint, especially in NMR and IR, gives clear cues for rapid quality assurance during synthesis or supply chain checks.
Suppliers provide 4-Acetylmorpholine with purity ratings ranging from 95% up to 99%, supported by certificates of analysis detailing NMR, GC-MS, HPLC or titration results. Labels emphasize CAS Number 1696-20-4, UN transport codes, hazard pictograms, and signal phrases required under GHS regulation. Many shipments include recommended drying and storage instructions, flagging the importance of keeping bottles tightly sealed and protected from light and moisture to prevent degradation. For institutions and industrial users, availability in bulk drums, drums with inner linings, and smaller volumetric bottles ensures flexibility, while batch-specific traceability wins trust from responsible procurement officers.
Many synthesis approaches draw on the acylation reaction between morpholine and acetyl chloride or acetic anhydride. Chemists charge a dry solvent, usually dichloromethane or toluene, and cool the mixture before slow addition of acetylating reagents, ensuring the temperature remains under control to avoid multiple substitutions or exotherms. Work-up steps include extraction, neutralization, and drying, yielding crude product that often requires distillation or chromatographic purification. For those who appreciate process runs, the yield commonly surpasses 80% with proper moisture and temperature management, and scale-up for industrial demand rarely introduces unexpected hurdles.
4-Acetylmorpholine's acetyl group opens up pathways for both nucleophilic and electrophilic attack. Its nitrogen atom can undergo further quaternization, while the acetyl group enables numerous condensation reactions. In peptide chemistry, labs sometimes use this molecule as a protecting group strategy or as an intermediate for more complex amide synthesis. Reductive reactions at the acetyl carbon or N-deprotection sequences see frequent application. Cross-coupling, alkylation, and other substitution patterns expand its library of derivatives, feeding demand in agrochemical and pharmaceutical sectors.
In catalogs and scientific reports, you might see names like N-Acetylmorpholine, 4-Morpholinyl methyl ketone, or 4-Ketoacetylmorpholine. While these aliases might cause confusion during literature reviews or procurement, the CAS number helps pin down the exact structure. In practice, suppliers from Europe, Asia, and North America stick to standardized nomenclature to minimize mix-ups in shipments or regulatory filings.
Proper lab practice demands gloves, eye protection, and handling under fume hoods due to the compound’s moderate skin and eye irritant profile. Safety Data Sheets (SDS) detail accidental spill protocols and first aid, while recommending tried-and-true PPE combinations. Chemists who have dealt with 4-Acetylmorpholine point out that it doesn’t pose the acute hazards seen with many acyl chlorides or highly reactive amines, but respiratory protection remains a must during bulk handling or during solvent-intensive reactions. Waste disposal follows organic hazardous waste streams, with labeling focus on mutagenicity testing and aquatic toxicity for regulatory compliance.
This morpholine derivative holds appeal for research groups in medicinal chemistry, where its basic nitrogen and carbonyl functionality support peptidomimetics, prodrug strategies, and lead compound optimization. Industrial polymer chemists have explored its inclusion for anti-static, emulsifying, or plasticizing functionalities. Its presence in agricultural chemical design draws from its stability and ability to carry functionalized moieties into soil or leaf environments. Analytical chemistry teams use 4-Acetylmorpholine as a reference standard or derivatization agent in assay development, taking advantage of its well-defined NMR and mass spectral signals.
University and industry researchers find 4-Acetylmorpholine a reliable stepping stone for testing new synthesis protocols, catalyst compatibility, and purification engineering. In drug development, teams build libraries of related structures for screening, benefiting from the compound’s ability to introduce controlled hard-soft acid-base characteristics. Scale-up specialists rely on existing literature yet tweak reaction kinetics to push cost and environmental efficiency, prompted by growing regulatory and sustainability scrutiny. New computational modeling continues to predict additional uses in organocatalysis and bioactivity.
Animal testing and cellular assays define 4-Acetylmorpholine as having moderate acute toxicity, with LD50 quantifications indicating that safe laboratory handling routines suffice. Genotoxicity panels, aquatic organism screening, and metabolism studies suggest less risk than halogenated morpholine derivatives, although chronic exposure remains under-studied. Researchers tracking occupational health outcomes find few if any systemic incidents tied solely to this molecule, yet compliance officers stay alert for any evidence of cumulative organ toxicity or carcinogenic breakdown products.
The morpholine scaffold remains a backbone of heterocyclic chemistry, and 4-Acetylmorpholine stands poised to gain new attention as green chemistry pushes for milder, more selective processes. Pharmaceutical companies continually hunt for building blocks that balance cost, reactivity, and toxicity; this molecule checks many of those boxes. Demand from precision polymer research, sustainable crop protection, and advanced analytical chemistry hints at steady growth. As requests for REACH-compliant, low-impact basic chemicals rise, 4-Acetylmorpholine is primed for new applications beyond traditional boundaries, provided ongoing research addresses any lingering toxicological questions.
A lot of lab regulars only recognize certain chemicals by their labels and warnings. But 4-Acetylmorpholine isn’t just a name on a bottle. Its molecular structure features a morpholine ring with an acetyl group attached. To most, that’s chemistry jargon, but the structure opens doors in research labs and factories.
4-Acetylmorpholine often shows up as a building block for other chemicals. Pharmaceutical companies know it as an intermediate—a step on the way to finished drugs or specialty molecules. The compound’s size, stability, and compatibility with other reagents let chemists build complicated molecules with fewer headaches. From what I've seen in the industry, using high-quality intermediates like this can keep entire production lines running.
Some manufacturers bring in 4-Acetylmorpholine when making agrochemicals. Pesticide and herbicide research always asks for something new or safer. Scientists evaluate many compounds along the way, and flexible intermediates like this one save time. Documented studies show its usefulness in synthesizing a wider range of target molecules, not just one big product line. Speed and reliability can mean the difference between winning and missing out on a market opportunity.
It surprises people to hear that flavors, dyes, and plastics sometimes involve 4-Acetylmorpholine. Stepping into an R&D lab, you notice the number of experiments built on chemical modifications. Add an acetyl group here, swap a substituent there—it all hinges on intermediates like 4-Acetylmorpholine. Access to these helps chemists try out new ideas without needing to reinvent everything from scratch.
Industrial solvents also draw from the morpholine family. Some formulas include acetyl derivatives. Anyone who’s worked on large-scale chemical production knows solvent choice affects everything from equipment corrosion to process yield. Reports from chemical engineering journals back up the claim—modifying solvents with specific functional groups, like acetyl, can tweak a process for better output.
Safety comes up every time a lab or plant worker handles new chemicals. 4-Acetylmorpholine shouldn’t be taken lightly. Material Safety Data Sheets (MSDS) provide the basics—wear gloves, avoid breathing the vapors, wash off any spills. Regulatory bodies such as OSHA list specific exposure limits for chemicals in the morpholine family, and for good reason. Mistakes can mean inhalation risks or skin irritation.
Environmental responsibility goes hand-in-hand with chemical handling. Industrial facilities adopting 4-Acetylmorpholine keep detailed records of waste and emissions. Proper disposal follows local and international rules, including those set by the EPA. Smart companies also evaluate greener alternatives or invest in closed-loop systems to minimize loss. Transparency isn’t just about avoiding fines—it builds trust with employees and with customers.
Industry insiders and researchers pay attention to both the potential and risks of 4-Acetylmorpholine. Chemists explore ways to streamline its synthesis, cut out more hazardous reagents, and reduce waste. Researchers keep sharing results in peer-reviewed journals, letting everyone stay up to speed on best practices and new findings. Keeping safety, quality, and transparency in focus helps prevent problems and ensures that innovations rest on a solid foundation.
4-Acetylmorpholine carries some interesting chemistry behind its structure. This chemical joins a morpholine ring with an acetyl group, showing up in industrial settings, research labs, and sometimes manufacturing. Even though it might not dominate headlines, more people interact with substances like this than most realize, especially in specialized labs around the world. Its use spans from solvent roles to intermediates in organic synthesis.
I’ve spent hours in labs where bottles with longer scientific names have much shorter warning labels. The safety of 4-Acetylmorpholine comes down to its handling rather than any flashy physical dangers. Current data shows that this compound does not cause fires or explode with the flick of a switch. Inhalation, direct skin contact, and accidental spills into eyes, though, pose the real concerns. Users report mild irritation when there’s poor airflow. Based on public safety data sheets, prolonged contact can dry out skin and may trigger mild respiratory discomfort if handled in large quantities or with poor face protection.
No evidence suggests that 4-Acetylmorpholine serves as a longstanding health threat like benzene or other notorious organics. Still, it pays to lean into caution. People rarely recognize a chemical hazard until something goes wrong—trust me, I’ve rinsed enough gloves and washed my eyes more than I intended to. Anyone who ditches gloves, neglects eye protection, or skips ventilation quickly realizes why those rules exist.
Good lab practices matter as much for 4-Acetylmorpholine as for any reagent. A chemical's lack of headline-making accidents should not make users relaxed. Carcinogenicity or mutagenicity usually pop up in paperwork if there is hard evidence, so most hazards here connect to mishandling or ignoring safety basics. My routine runs like clockwork: gloves on, goggles tight, fume hood down. Separate labeled waste containers and regular reviews of safety data sheets create a culture where near-misses drop sharply.
Stuff does spill sometimes. Laboratories that encourage immediate incident reporting handle accidents faster and prevent a headache later. Quick access to eyewash stations, lab coats, nitrile gloves, and well-maintained exhaust systems puts the odds in your favor every day.
One danger comes when small risks pile up unnoticed. 4-Acetylmorpholine might not make news for mass poisoning events, but neglect can lead to chronic irritation, lab closures for fumigation, or lost research material—costs no one wants. Routine storage checks, labeling protocols, and training programs keep small issues from growing.
People often trust themselves to 'know their chemicals.' Unfortunately, confidence leads to corner-cutting. Google’s guidelines encourage accurate, timely, and reliable information, placing the user’s health and well-being first. Mistaking a mild irritant for a safe material puts people in harm's way, so reviewing primary sources, independent government warnings, and the manufacturer’s safety data offers the best shield.
Storing 4-Acetylmorpholine inside tightly sealed bottles, away from acids or bases, deters surprise reactions. Fresh gloves, closed-toe shoes, and eye protection help every person working with any chemical, whether an expert or new trainee. Avoiding direct sniffing or tasting may sound obvious, but emergencies have shown it’s worth repeating. Regular training and safety reminders don’t just hit compliance checkboxes—they build habits that protect everyone in the lab environment.
By treating every chemical with respect—and a bit of healthy skepticism—labs hold onto both safety records and peace of mind.
Start with the basics: 4-Acetylmorpholine carries the molecular formula C6H11NO2. The structure centers around a morpholine ring, which includes both an oxygen atom and a nitrogen atom set into a six-membered ring. Imagine a house with two doors—a nitrogen and an oxygen—giving this molecule its unique entry points for reactivity and functionality. At the fourth position on that ring, there’s an acetyl group, which is a simple addition of a carbonyl (C=O) with a methyl group (CH3).
My first brush with 4-Acetylmorpholine happened while interning in a pharmaceutical lab, where we focused on small modifications to common ring structures. This compound’s structure made it ideal for exploring new solvents and chemical syntheses because it straddles the line between water-loving (hydrophilic) and oil-loving (lipophilic) features. The morpholine ring gives it that water-compatibility, while the acetyl punch adds a bit of oil-friendly twist, broadening how it plays with other chemicals.
People often overlook how small tweaks on a chemical ring can flip its behavior completely. The acetyl on the morpholine transforms its reactivity and how it partners with other compounds. Instead of just being a benign ring found in everything from analytical testing to drug synthesis, 4-Acetylmorpholine can step up as a building block for more complex molecules. Medicinal chemists use it precisely because that little acetyl tag changes how the molecule fits into biological environments or chemical reactions. Data from recent journals back this up—research shows substituting different groups at the fourth position drastically impacts the molecule’s solubility, toxicity, and interaction with enzymes.
Chemistry students sometimes think it’s only the big changes that matter. I learned the opposite. During a graduate lab, we tried switching out groups at the fourth position on morpholine. Swapping in that acetyl altered pharmacokinetics and how the molecule broke down inside cells. Small details, like a carbonyl’s electron pull or a methyl’s bulkiness, can decide whether a drug clears infection or stays inert.
Industrial teams appreciate 4-Acetylmorpholine because it’s often safer and easier to handle than nastier solvents or reagents. Safety data sheets show it has relatively low acute toxicity and needs only basic protective measures. Still, no one wants to ignore its possible irritant effects or keep it uncontained in lab settings. Practical chemistry isn’t about bravado; it’s about understanding which gloves and goggles to wear so that routine exposure doesn’t sneak up over years.
If you work with this molecule regularly, proper ventilation and short exposure times make a difference. National agencies like the U.S. National Institute for Occupational Safety and Health (NIOSH) provide clear guidelines. Listening to experienced lab techs and checking up-to-date documentation helps keep everyone healthy.
Every compound tells a story through its structure. 4-Acetylmorpholine represents how small, intentional changes in chemical design can create safer and more versatile molecules. Understanding these details fosters trustworthy science and smarter choices, whether inventing new treatments or running safer lab processes. The lesson remains: respect the structure, and the chemistry will follow.
Storing chemicals like 4-Acetylmorpholine often crosses over into real-world safety concerns. In my own experience working in small research labs, poor chemical storage habits can create more problems than anyone expects. Bottles tucked into odd corners without clear labeling leads to confusion. Sometimes, containers go missing. People forget they ever handled a substance. Before long, the space stops being safe for work. Simple, practical steps help avoid these headaches.
4-Acetylmorpholine reacts to conditions around it. Elevated temperatures, direct sunlight, and open-air exposure can shift its chemical stability. Cool storage conditions help. Every reputable safety data sheet for this compound points toward temperature ranges close to 2°C to 8°C (refrigerator cold works well for most research settings). Keep it away from heat sources like windows or radiators.
Moisture represents a silent threat. Humid environments slowly break down some organic chemicals, even if nothing looks wrong at first glance. In shared lab refrigerators, moisture can sneak in every time someone grabs lunch. Airtight containers, such as glass bottles with secure caps or sturdy plastic vials, cut this risk. For years, I watched colleagues ignore this advice—resulting in strange smells and crystal formation inside jars. It never ended well.
4-Acetylmorpholine doesn't mix well with strong oxidizers and acids. Keep those shelves separate. Rows of well-labeled containers make a big difference: clear, waterproof tape and bold, legible writing become your best friends. Always include the full chemical name, concentration, and date received. Over time, handwritten scribbles fade or smudge. Printed labels work better.
I’ve seen many students grab the wrong bottle by mistake, simply because someone reused a container and forgot to update the label. These preventable errors add unnecessary risk. Signs on storage units and clear inventory lists go a long way in both academic and industrial environments.
Safety isn’t a one-time job. Regular inspections of chemical stock—every three to six months in a busy lab—can catch spilled powders, broken caps, or expired containers long before an accident happens. I once worked in a school where neglected vials leaked, causing a sticky mess and a day-long shutdown. Checking inventory and logging removal or disposal avoids this.
Having proper spill containment materials nearby saves time and trouble. Simple things help: an absorbent pad, waste bags, gloves, and safety goggles. Staff should know where to find them without turning the storage room upside down. Emergency contact information for chemical exposure must hang on the wall, not buried in a folder somewhere.
Every lab handling 4-Acetylmorpholine eventually faces disposal questions. Dumping unused material down a drain or in general waste bins breaks environmental law and can harm water supplies. Follow your local hazardous waste procedures. Regularly scheduled waste pickup, with chemicals segregated by class, builds an effective safety culture.
Practical storage of 4-Acetylmorpholine comes down to common sense, supported by formal training and good routines. Experience repeatedly teaches that shortcuts on safety end up costing more in time, money, and health than people anticipate. Safe storage keeps work moving and protects everyone sharing the space.
People often overlook smaller, specialty chemicals behind the scenes. I’ve come to appreciate 4-Acetylmorpholine not because it attracts headlines, but because it gets work done without fuss or drama. This compound, part of the morpholine family, earns trust from chemists for reliability in various industrial tasks.
In my experience, industries don’t chase after trendy chemicals just for novelty. They focus on what works and what’s proven. 4-Acetylmorpholine gets most of its attention in three key areas: solvent use, intermediate in synthesis, and formulation aid.
Solvent WorkhorsePaint and coating companies depend on stable, versatile solvents for consistent results. 4-Acetylmorpholine steps in as an effective co-solvent. It helps dissolve and blend dyes, pigments, and specialty chemicals. This raises color fastness in textile dyes or avoids streaking in finished coatings. I’ve seen textile dyeing processes grind to a halt when lower-quality solvents are used, leading to ruined batches and lost revenue.
Stepping Stone in SynthesisPharmaceutical teams, especially those focused on research and development, use 4-Acetylmorpholine as a building block. Medicinal chemists draw on its structure to assemble compounds with pain-relief or anti-inflammatory properties. Custom molecule design sounds high-brow, but it really boils down to having reliable intermediates. In my conversations with lab managers, they always mention purity and consistent reaction performance as the reasons they keep 4-Acetylmorpholine in their inventory.
Formulation Challenges Made EasierIn agrochemicals and specialty lubricants, recipes can get complicated. Small tweaks in chemical structure sometimes make the difference between a product working as promised or failing in the field. 4-Acetylmorpholine finds its niche here by stabilizing active ingredients or improving the final blend’s storage properties. I know QA engineers who swear by it, citing how it keeps mixtures from breaking down or separating during transport.
Every company looks for cost, reliability, and safety. 4-Acetylmorpholine isn’t hazardous in small-scale use, which makes it attractive for busy production environments or crowded lab benches. Regular operators find it easy to handle under basic chemical safety protocols. Accidents are rare when professionals follow established procedures.
Supply chain managers keep a keen eye on consistency. No one wants delayed shipments to grind operations to a halt. Reputable chemical suppliers maintain robust protocols for 4-Acetylmorpholine quality and traceability. Companies worldwide rely on this because downstream errors mean time and money lost.
The chemical industry always faces pressure to use resources more efficiently and reduce environmental impact. 4-Acetylmorpholine, with its stability and multi-purpose nature, helps manufacturers avoid waste. By sticking to compounds that offer safe, predictable results, industries cut down on recalls, rework, and disposal costs. Teams working towards greener operations keep integrating such chemicals into processes that require fewer cleanups or lower emissions.
From laboratories to large-scale manufacturing, people in the know appreciate 4-Acetylmorpholine’s steady performance. Whether it’s helping paint shops achieve perfect finishes or supporting pharmaceutical research, this chemical proves that strong results come from the right tools—often the ones people don’t notice most.
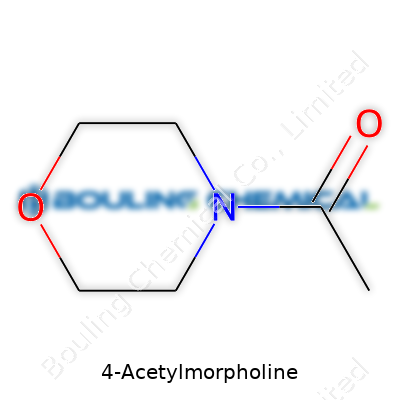
| Names | |
| Preferred IUPAC name | 1-(Morpholin-4-yl)ethan-1-one |
| Other names |
4-Morpholinyl methyl ketone 4-Morpholineacetone 4-Acetyl morpholine 1-Acetyl-4-morpholine |
| Pronunciation | /ˈfɔːr əˈsiːtɪl mɔːˈfɒlɪn/ |
| Identifiers | |
| CAS Number | 1696-20-4 |
| Beilstein Reference | 1209244 |
| ChEBI | CHEBI:139765 |
| ChEMBL | CHEMBL40968 |
| ChemSpider | 71021 |
| DrugBank | DB08319 |
| ECHA InfoCard | 100.010.095 |
| Gmelin Reference | 8059 |
| KEGG | C01724 |
| MeSH | D019292 |
| PubChem CID | 12009 |
| RTECS number | SE2975000 |
| UNII | 48LFW7BH11 |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C6H11NO2 |
| Molar mass | 143.18 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Weak, fruity |
| Density | 1.101 g/mL at 25 °C (lit.) |
| Solubility in water | Soluble |
| log P | -0.41 |
| Vapor pressure | 0.0267 mmHg (25°C) |
| Acidity (pKa) | pKa = 15.71 |
| Basicity (pKb) | 5.85 |
| Magnetic susceptibility (χ) | -5.54 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.485 |
| Viscosity | 1.84 mPa·s (20°C) |
| Dipole moment | 4.22 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 325.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -482.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2257.6 kJ/mol |
| Pharmacology | |
| ATC code | N02AX13 |
| Hazards | |
| Main hazards | Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 94 °C |
| Autoignition temperature | 260 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat): 860 mg/kg |
| LD50 (median dose) | LD50 (median dose): 700 mg/kg (oral, rat) |
| NIOSH | NIOSH DU8225000 |
| PEL (Permissible) | PEL (Permissible) for 4-Acetylmorpholine: Not established |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
N-Methylmorpholine 4-Methylmorpholine 4-Formylmorpholine Morpholine 2-Acetylmorpholine |