Interest in pyrrolidine derivatives picked up sharply after the middle of the twentieth century, especially once scientists started learning more about nitrogen-containing heterocycles. Back then, much of the progress was driven by the search for pharmaceutical leads and new building blocks for organic synthesis. 4-Acetyl-pyrrolidine surfaced as chemists explored new tweaks of pyrrolidine’s structure, hunting for specialized reagents and intermediates. Over years, researchers tried multiple routes to attach different functional groups around the pyrrolidine ring. Finding a reliable lab-scale method for acetylation at the 4-position became a marker of progress in synthetic methodology. Several generations of chemists tinkered with reaction conditions, solvent choices, and temperature controls to get the kind of selectivity they needed. Today, the compound stands out for its role as a trusted intermediate in producing other useful chemicals, and its development traces a story of steady trial, error, and tenacity in organic labs.
4-Acetyl-pyrrolidine lands in chemical catalogs as a niche but valued product. Not the sort of compound grabbing headlines, but ask anybody in medicinal chemistry or materials R&D and you’ll hear about its usefulness as a versatile building block. The compound serves a solid purpose as a starting material for molecules aimed at the pharmaceutical, agrochemical, and specialty polymer sectors. Researchers often seek it out because the acetyl group at the fourth position gives an entry site for further modifications, while the pyrrolidine ring brings stability and reactivity. Most commercial supplies appear as colorless to slightly yellowish liquids or low-melting solids, presented in well-sealed containers to avoid moisture uptake and oxidation. Chemists see 4-acetyl-pyrrolidine as a backbone for more complex molecules, and its commercial profile centers on consistency, purity, and reliable supply.
A glance at the structure hints at a clear direction for its physical and chemical traits: the five-membered pyrrolidine ring shapes its volatility, solubility, and reactivity, while the acetyl group at the fourth spot introduces an electron-rich carbonyl. Most suppliers report a boiling point in the lower-to-mid 200s °C, making it manageable in standard fume hoods but still requiring care against evaporation. Density sits just under that of water, and solubility runs high for most organic solvents but hovers at moderate levels for water. Chemists highlight its sensitivity toward strong oxidizing agents and recommend storing it in cool, dry conditions. The carbonyl group boosts its profile as a nucleophilic acceptor, inviting additions and condensations. Any work with 4-acetyl-pyrrolidine in the lab needs a good grasp of its moderate volatility, potential for amide or imine formation, and its resilience against base-catalyzed degradation.
Companies shipping 4-acetyl-pyrrolidine generally stick to high-purity lots—think 97% and above—targeting research and industrial buyers. Labels on containers state batch number, concentration, and date of manufacture, with extra detail about possible impurities such as residual solvents or similar pyrrolidine derivatives. Standard safety labels warn against skin and eye contact and highlight any necessary emergency measures. Documentation arrives with updated safety data sheets, along with information about shelf life and conditions that help preserve purity. Reliable companies often verify quality using a blend of NMR, GC-MS, and IR, and this analytical data comes with the shipment. While regulations about storage and disposal follow country or state rules, reputable sellers stress tightly closed vessels and cool storage as best practices. Clear labeling matters, since a single mix-up between different acetylated rings could derail a whole research batch.
There’s no single route to this compound, but the preferred one usually involves acetylation of pyrrolidine or one of its derivatives. In the most straightforward process, chemists start with pyrrolidine and use acetic anhydride, sometimes with catalytic acid or base, to attach the acetyl group at the 4-position. Control during the reaction becomes key: too much heat or the wrong catalyst encourages acetylation elsewhere, including the nitrogen. Many labs experiment with protection-deprotection cycles to shield reactive sites, sometimes using blocking groups on the nitrogen before targeting C-4. A round of purification follows, relying on distillation or recrystallization to weed out isomers and starting material. Some teams swap out acetic anhydride for acetyl chloride, or use solvents like dichloromethane or toluene, depending on scale and available equipment. This focus on targeted modifications reflects the journey chemists take—testing variable after variable until they reach the cleanest conversion with the least waste.
The beauty of 4-acetyl-pyrrolidine for a synthetic chemist lies in that open carbonyl group. The molecule behaves as a nucleophilic partner in various condensation reactions, letting chemists build up bigger rings or linkages with relative ease. Reductive amination using mild reducing agents produces N-substituted derivatives for pharmaceutical work. Given the structure, the carbonyl group also opens doors for aldol-type additions, which feed right into the creation of more elaborate nitrogen heterocycles. Reduction with hydride donors like sodium borohydride turns the acetyl into an ethyl, sometimes as an intermediate step in total synthesis. Some researchers report oxidative transformations at C-4 which broaden the array of accessible analogs. Efforts in academic labs keep the chemistry around this core structure lively, since each new connecting point means new possibilities for modifying biological activity or physical properties.
Searching for this compound in chemical databases brings up several synonyms worth knowing. Names such as 1-Pyrrolidineethanone, 4-oxo-pyrrolidine, and NSC 37063 frequently appear in literature and supplier catalogs. International sourcing often brings up language variants or structural descriptors—sometimes just “4-acetylpyrrolidine” and sometimes more systematic approaches like “1-(Pyrrolidin-4-yl)ethanone.” Regulatory documents and Safety Data Sheets list CAS Number 932-16-1 to help avoid confusion. A few custom manufacturers trade under their own codes or trademarks, but academics and industrial chemists tend to stick to these systematic or truncated names for clarity.
Handling 4-acetyl-pyrrolidine demands strict adherence to chemical safety. This compound brings moderate skin and eye irritancy, and inhalation of vapors can cause discomfort or more severe symptoms with prolonged exposure. Proper use of gloves, safety goggles, and fume hoods sits at the foundation of safe handling, and spill kits should always be handy in any lab working with nitrogen heterocycles. The chemical’s storage requirements call for cool, well-ventilated areas away from oxidizers or sources of ignition. Disposal runs through established routes for organic solvents and amines, with careful neutralization and downstream wastewater monitoring. Company protocols and institutional guidelines both reinforce personal responsibility and regular safety training. Labs using this material often conduct safety drills and keep Material Safety Data Sheets accessible. Regularly reviewing the toxicology profile ensures workers stay alert to acute and chronic exposure risks.
Chemists working on new drug scaffolds gravitate toward 4-acetyl-pyrrolidine. Its molecular shape and balanced reactivity make it a go-to for constructing lead compounds aimed at treating neurological and psychiatric disorders. Agrochemical R&D teams value its ability to serve as a starting point for selective herbicides or growth-regulating agents. Beyond pharmaceuticals and agrichemicals, some polymer engineers use it as a monomer for specialty polyamides or for tuning the flexibility of new materials. Research outfits investigating catalysis tap its basic nitrogen and carbonyl chemistry to develop advanced ligands and organocatalysts. The compound’s adaptability across research disciplines shows its broad relevance and keeps it in steady demand for both method development and end-product manufacturing.
The story of R&D around this compound keeps expanding, with researchers focusing on greener synthesis methods, improved selectivity, and new derivative compounds with tailored properties. In the last decade, a clear trend points towards mild reaction conditions using less hazardous solvents and reagents. Some academic groups explore catalytic alternatives to traditional acetylation, pushing for higher yields and lower waste. Others hone in on developing libraries of substituted pyrrolidines using this compound as a modular intermediate, screening them for activity against different disease targets. Collaborations between university labs and pharmaceutical companies drive much of the innovation, especially where rapid analog synthesis stands to yield breakthroughs in drug discovery. Publications covering analytical method improvements—such as rapid HPLC assays or new NMR techniques—keep expanding, fueled by the relentless pace of demand from organic chemists.
Toxicity data for 4-acetyl-pyrrolidine remains limited compared to mainstream industrial chemicals, but the compound’s relatives within the pyrrolidine family have informed expectations for acute and chronic risks. Rodent studies flag moderate toxicity at high doses, with metabolic breakdown involving liver enzymes known to handle simple amines and amides. Long-term exposure data isn’t abundant, but chemical safety officers point to the need for regular monitoring and strict exposure limits, particularly in poorly ventilated spaces. Some preclinical toxicology efforts track metabolic pathways to make sense of the buildup of potentially reactive intermediates. Environmental impact assessments suggest low persistence but urge careful disposal, citing possible effects on aquatic life if released in bulk. Calls for more rigorous, transparent reporting of in vivo and in vitro toxicity continue to rise, especially as more research groups build bigger libraries of related heterocyclic compounds.
Expect the future for 4-acetyl-pyrrolidine to center on eco-friendly manufacturing, expanded pharmaceutical applications, and better safety data. Green chemistry principles guide efforts to swap out hazardous solvents and minimize waste, with pilot plants testing continuous-flow methods and novel catalysts. Drug development projects increasingly prize the compound for its reliability as an intermediate, while polymer science teams eye it to build new biomaterials with unique mechanical traits. The growing interest in heterocyclic scaffolds keeps labs busy tweaking its structure for targeted effects in everything from brain health to crop resilience. Widespread adoption will demand more comprehensive toxicity research and cross-sector regulatory dialogue. Demand for process improvements—like lower-energy synthesis or solvent recycling—drives ongoing technical work. The push for smarter, cleaner products means chemists, toxicologists, and engineers will keep returning to 4-acetyl-pyrrolidine as both a challenge and a platform for discovery.
4-Acetyl-Pyrrolidine isn’t something you’ll find at the corner pharmacy or stacked on the shelves in a regular grocery store. At its core, it’s a chemical compound—a clear or slightly yellowish liquid, and it shows up in plenty of research labs and industrial settings. The casual observer might think it's just another complex name in a sea of chemicals, but it actually plays a role in many industries most people interact with, even if they don’t realize it.
Pharmaceutical researchers prize this compound for how it acts as a building block. Drug companies want molecules that can snap together in dozens of ways, and 4-Acetyl-Pyrrolidine fits right in thanks to its structure. Chemists use it for making intermediates—complex steps bridging basic materials to finished medicines.
The best way to explain the value here is to look at its role in developing new drugs for the central nervous system. Families of molecules related to pyrrolidine often help scientists work toward treatments for depression, schizophrenia, and other brain-related illnesses. It’s puzzling how little everyday folks realize the footsteps behind their daily pills sometimes trace back to ingredients like this.
Crops don’t protect themselves. Farmers need products to keep pests and diseases at bay, so chemists step in. Compounds built around pyrrolidines have made their way into modern pesticide production. By tweaking molecules with parts like 4-Acetyl-Pyrrolidine, researchers can invent new pesticides or improve the action of old ones. Some newer herbicides and fungicides owe their punch to chemical designs involving this molecule.
Crop loss from pests and blight takes a chunk out of global food production every year. Chemicals like these—controversial as the topic may be—end up playing a hidden role in getting food on the table, making the link between tricky organic chemistry and your morning bowl of cereal closer than most people might guess.
Industrial chemists think about efficiency and precision. Gathering the right basic molecules means less waste and more predictable results. 4-Acetyl-Pyrrolidine acts as a sort of foundation in the assembly of specialty chemicals. The value shows up in speeding up reaction times or unlocking new reactions that older building blocks can’t manage.
Let’s say the goal is to invent a new rubber, glue, or dye. With its unique balance of nitrogen and carbon atoms, this compound lets engineers design materials that last longer or perform under tougher conditions. Materials made with components like these show up in items spread around modern life—think advanced coatings or specialty plastics.
Once a molecule like this lands in multiple supply chains, safety questions follow. Handling chemicals in factories or research labs comes with rules for ventilation, gloves, and eye protection. Regulatory agencies keep updating safe usage guidelines, aiming to keep workers out of harm’s way. Companies can’t just chase efficiency—they have to test for toxicity and environmental impact. With more firms seeking “greener” alternatives, pressure grows to design similar compounds that break down into harmless byproducts.
4-Acetyl-Pyrrolidine might not make headlines, but it sits behind some of the most active lab benches in the world. Without these chemical building blocks, big jumps in medicine, agriculture, and materials science would slow down or stop. Next time you reach for a packaged snack or pick up a prescription, a behind-the-scenes compound like this may already have traveled the scientific road that made it possible.
4-Acetyl-Pyrrolidine comes across as an off-white to pale yellow solid, often showing up in labs in small crystalline bits or powder. This isn’t a substance that grabs your nose straight away, but if you open a fresh container, there’s a faint, sweetish scent—something faintly chemical, nothing that’s going to trigger memories of fruit. It's not what you’d call highly volatile, and it doesn’t dissolve well in water. The reason for that low solubility? The acetyl group gives the molecule a slightly greasy texture—it finds more comfort in organic solvents like ethanol, ether, or chloroform.
Melting point checks usually fall somewhere between 51°C and 54°C. Change out the room temp for a local heat source, and it’ll shift from solid to a clear, colorless liquid without making a mess. Trying to burn or heat it harshly isn’t wise: similar compounds tend to break down into smoky, pungent products, and you don’t want that in a small space. In my own stints measuring melting points, pyrrolidine derivatives like this one never give a sharp, textbook melting point—expect a couple of degrees’ worth of “slush” before they really liquefy.
At its core, this molecule brings together a five-membered nitrogen ring (pyrrolidine) and an acetyl group. That pairing changes the game in small but important ways. Pyrrolidine by itself is pretty reactive—it’s basic, picks up protons easily, and finds its way into everything from synthetic pharmaceuticals to rubber. Add the acetyl group, and suddenly that nitrogen doesn’t act as eager. The lone pair on the nitrogen interacts with the acetyl carbon, dialing back basicity. In day-to-day chemistry, that lowers the risk of surprise reactions, so you can use it in selective syntheses where less-reactive amines are handy.
You can still tug some chemistry out of it. Acetyl groups can come off with enough heat and the right acid or base. Overall, though, it stands up well to everyday air and moisture. People storing small samples in clear vials outside the fume hood find it keeps for months without turning brown or sticky. That stability matters for research labs—nobody wants a key compound transforming on the shelf. It’s not going to pick up water from the air, it stays solid unless you push it with heat.
Chemists value 4-Acetyl-Pyrrolidine for its balance between reactivity and stability. In my own work, using pyrrolidine rings as stepping stones toward more complex molecules, tacking on an acetyl group can protect the nitrogen until you’re ready to use it. Synthetic organic chemistry, especially for pharmaceutical work, leans hard on small modifications like this. It helps direct the chemistry down narrow paths, letting you reach tight targets, like the precise placement of a drug’s key functional group.
That doesn’t mean it’s without risk. The relative blandness of its solid form, coupled with its low vapor pressure, can cause people to get sloppy. I’ve seen more than one rookie forgo gloves, thinking “it doesn’t smell, must be harmless.” Not a wise move. Pyrrolidines can soak through skin, and while toxicity data for the acetylated version isn’t as scary as other amines, it’s smart to treat them with respect.
Laboratories and chemical suppliers would do well to improve their safety sheets specifically around these types of compounds. Blanket statements lumping all pyrrolidines together ignore the subtle differences that come from acetylation. A push for clearer labeling and better storage guidelines could cut down on accidents. For researchers, spending a little more time understanding how a small tweak like acetylation changes both handling and reactivity pays off in waste reduction and lowered risk.
I’ve watched labs pay the price for choosing convenience over care. Chemical spills stick in my mind, not just for the cleanup, but because people often ignore how those accidents start. 4-Acetyl-pyrrolidine doesn’t draw headlines like more notorious compounds, but it brings its own risks if someone shrugs it off as routine. This isn’t just a matter of organization—the real issue involves health, safety, and expense when things go wrong.
If you crack open a bottle without clear labeling, cluttered shelves, or missing documentation, someone’s likely to make the kind of mistake that leaves you wishing you’d taken five extra minutes upfront. Some see storage as a chore, yet the alternative often costs labs equipment, money, or even someone’s well-being.
A lot of labs use metal or plastic cabinets for chemical storage, but not every cabinet works for every chemical. With 4-acetyl-pyrrolidine, a solid, locked cabinet—high enough off the floor to avoid flood risks—usually makes a good pick. Water leaks aren’t a small concern, especially for folks in old buildings.
Keep this chemical away from direct sunlight. Watch the temperature. Excess heat causes pressure build-up in bottles, leading to leaks or worse. Choose a cool, dry place and keep it separate from acids, oxidizers, and strong bases. One mistake some people make is thinking “as long as it’s capped, it’s safe anywhere.” That confidence fades the day two incompatible bottles get knocked together and react.
Keep every container labeled with purchase or open dates, name, and hazard symbols. If you’ve ever stumbled over a mystery bottle during an inventory, you remember the hassle and risk. A barcode or QR system can help, but there’s no substitute for a plain label.
Every glove isn’t equal. Nitrile gloves handle 4-acetyl-pyrrolidine better than latex or vinyl. Safety glasses might seem like overkill until the day someone shakes a bottle too vigorously and gets a splash. I’ve seen good people get sidelined from work because safety goggles felt “annoying” just for a second.
Work under a chemical fume hood with good airflow. Ventilation isn’t a luxury—it’s the difference between a minor odor and an air quality problem. If you can smell anything while working, the setup needs work. Spills call for absorbent pads and a spill kit, not just paper towels. Skin contact means washing up right away—soap and water beat any cleaning shortcut.
Never eat, drink, or store food near chemicals. Cross-contamination turns a minor mistake into a real emergency. Sturdy, closed-toe shoes and long pants do more than look professional—they protect your skin from splashes and dropped bottles.
Training sounds like red tape, but skipping it is a gamble. Teach new folks with real examples and encourage them to ask questions, not just memorize rules. Everyone should know where to find the SDS and understand hazard symbols. Keep track of inventory with regular audits. Running out of a chemical is inconvenient, but finding an expired or damaged bottle brings bigger headaches.
Reporting unsafe conditions needs open discussion. Skipping over a broken label or a rusty cap leads to someone else facing the risk later. Building a climate where people speak up, even about small details, helps steer clear of preventable problems.
Good storage and handling come down to habits. Designate a storage spot and stick to it. Rotate older stocks to the front, just like in a grocery store. Hold short safety check-ins every week—five minutes pays off in avoided incidents. Rely on people as much as systems. You can have all the signs and policies in the world, but everything picks up dust if the culture doesn’t value speaking up about risks.
People often underestimate the risks attached to working with and around industrial chemicals. Take 4-Acetyl-Pyrrolidine for example—a compound that comes up in labs and manufacturing setups. Stepping into these environments, even with a bit of know-how, it’s clear that safety goggles and gloves aren’t just for looks. Each chemical carries its own story of potential trouble, and this one isn’t any different.
Ask anyone who works hands-on with small molecule synthesis—skin contact feels minor until you see redness or rash where none existed an hour before. Exposure to 4-Acetyl-Pyrrolidine may result in these symptoms. More worrying, chemicals that don’t sting or smell particularly strong can still sneak through layers of unprotected skin. Eye exposure is even riskier, causing stinging or even clouded vision. Even after working in academic research, I’ve seen folks downplay spills and then regret it later, rushing to eye-wash stations.
The story doesn’t end with physical contact. Fumes from 4-Acetyl-Pyrrolidine rarely fill a room with a warning odor, tricking people into thinking the air’s safe. If you’ve spent time in a poorly ventilated lab, you know the strange dizziness or dry cough that creeps up after an hour or two. Studies on similar compounds highlight respiratory irritation, headaches, and even nausea at heavy exposures. Working with any volatile solvent without proper hoods or masks rolls the dice with your airways.
Accidental ingestion happens far more often than textbooks admit—a hand pops a sandwich into a mouth before a proper wash. Swallowing 4-Acetyl-Pyrrolidine or residues can cause nausea, abdominal pain, and, in larger amounts, more serious troubles with the gut or nervous system. Data on this chemical class suggest the body doesn’t clear these molecules quickly, so the problems stick around longer than one might hope.
Longer careers around chemicals accumulate risk. Chronic exposure, even at low levels, can bring up issues like sensitization—where someone develops allergies after handling the same substance again and again. Although detailed studies on this molecule lag behind more famous toxins, comparing to similar pyrrolidine chemicals points toward liver and kidney stress over months or years. Several cases from older labs report workers developing fatigue or odd lab test results, only to trace it back to workplace air quality and routine contact.
Ignoring storage and spill protocols can bring fire hazards. 4-Acetyl-Pyrrolidine won’t erupt on contact with air, but flammable properties come into play with high temperatures or open flames. I remember seeing small solvent fires start in under-supervised labs. Rapid cleanup, good labeling, and strict inventory habits make a big difference. Spills need absorbent materials handy nearby, not forgotten in a distant supply closet.
Lab safety shouldn’t rely on warning labels alone. Regular checks on ventilation, using gloves that aren’t worn thin, and keeping eye protection within arm’s reach: these habits are built from the stories of people who ignored them. Documenting near-misses and sharing lessons makes the whole workplace smarter. Substitution with less toxic alternatives counts, too. Before any shipment or new project, reviewing newer safety data sheets (SDS) matters more than ever; industry guidelines don’t always keep up with new findings.
Safety never finishes with one poster on the wall. The habits that reduce hazards with 4-Acetyl-Pyrrolidine benefit from a culture where everyone calls out risky behavior. With more attention to the risks, fewer accidents slip through the cracks, and the invisible toll of chemical exposure stays lower.
Purity isn’t just a number on a label, especially with chemicals like 4-Acetyl-Pyrrolidine. This compound usually shows up at around 98-99% purity in most legitimate supply chains. That trace percent may not sound like much, but in chemical manufacturing or research, it decides whether your product performs as expected or not. Impurities clog up processes, change reaction pathways, or just deliver inconsistent results. In my own work with fine chemicals, I’ve seen how even a small bump in purity can be the difference between a reliable outcome and a headache that drags on for weeks.
Producers advertise high purity, but lab analysts know brands often mean slightly different things by those numbers. Some suppliers include moisture content in their percentage, while others factor in residual solvents. It pays to read the Certificate of Analysis before trusting what’s in the bottle. Analytical methods for testing—HPLC, NMR, or GC—should always back up the supplier’s statement. I’ve found too many research projects hit a wall due to buyers skipping this diligence. With sensitive projects, checking a lot or two directly at your lab provides peace of mind.
It’s interesting to see how packaging tells you who the real end-user is. Academic settings ask for 10-gram, 25-gram, or 100-gram amber glass bottles. This keeps small-scale experiments manageable, reducing waste and making it easy for postgrads to handle the compounds without much fuss. Those tiny screw-cap bottles sit neatly in refrigeration units and containment cabinets.
Industrial customers think bigger, hunting for half-kilo and kilo containers, sometimes up to 5 kg in a single drum. Polyethylene jugs and HDPE barrels start to appear, because heavy glass risks breakage in the warehouse and during shipping. Bulk orders bring lower price-per-gram but raise other issues. Companies need extra record-keeping for regulatory reasons and better systems for sealing off partially used inventory. My own experience tells me improper repackaging or poor seals can lead to moisture leaching in or cross-contamination—costly lessons for anyone unwrapping an expensive container six months after it was opened.
You’ll rarely see casual packaging, at least from reputable suppliers. Most institutions prefer tamper-evident seals. Labeling is precise: chemical structure, batch number, production date, and hazard markings. In Europe or North America, suppliers follow REACH or OSHA standards, so nothing gets left to guesswork. The right labeling has saved me more than once in the lab, especially wrestling with similar-looking bottles on a crowded shelf.
Getting 4-Acetyl-Pyrrolidine in the correct purity and container makes lab life simpler and safer. Bad packaging might let sunlight or air degrade your compound before you’ve even run a single test. Any mistake here swings costs, increases chemical exposure, or just ruins a research week. Roughly a decade back, my team ran a synthesis with what turned out to be off-spec material; only after reordering from a trusted source did the reactions finally behave.
Tighter controls on packaging and purity guard against shipping accidents, theft, or legal snags. Newer packaging innovations, like single-use sachets or vacuum-sealed liners, are helping smaller labs squeeze more out of purchases without worrying about leftovers spoiling or contaminating other materials.
If trouble still comes knocking, direct lines to the manufacturer or distributor help solve it faster. Companies that offer live batch tracking and digital Certificates of Analysis as part of their shipping process get repeat business. This sort of reliability keeps research labs, factories, and universities running smoother—something anyone who’s ever had a project derailed will appreciate.
| Names | |
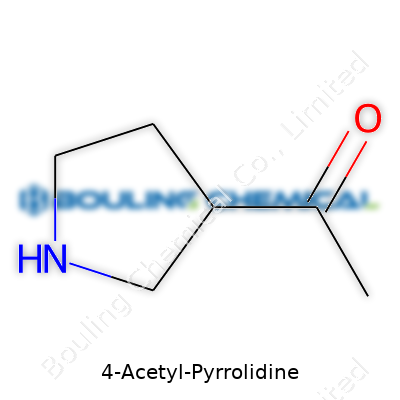
| Preferred IUPAC name | 1-(Pyrrolidin-4-yl)ethan-1-one |
| Pronunciation | /fəˈræs.ɪl paɪˈrɒl.ɪˌdiːn/ |
| Identifiers | |
| CAS Number | 2418-75-9 |
| Beilstein Reference | 1109280 |
| ChEBI | CHEBI:189643 |
| ChEMBL | CHEMBL1876608 |
| ChemSpider | 142373 |
| DrugBank | DB08242 |
| ECHA InfoCard | 100.063.827 |
| EC Number | 624-14-4 |
| Gmelin Reference | 112209 |
| KEGG | C18827 |
| MeSH | D04717 |
| PubChem CID | 11671441 |
| RTECS number | UY4375000 |
| UNII | 2X8JT02D3D |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C6H11NO |
| Molar mass | 113.16 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | amine |
| Density | 1.06 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | -0.31 |
| Vapor pressure | 0.205 mmHg (at 25 °C) |
| Acidity (pKa) | pKa = 11.3 |
| Basicity (pKb) | 2.94 |
| Magnetic susceptibility (χ) | -47.7·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.482 |
| Viscosity | 0.948 cP (25°C) |
| Dipole moment | 2.85 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 359.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -204.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4397.6 kJ/mol |
| Pharmacology | |
| ATC code | No ATC code assigned. |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and serious eye irritation |
| GHS labelling | GHS02,GHS07 |
| Pictograms | GHS05, GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0-NULL |
| Flash point | 76°C |
| Lethal dose or concentration | LD₅₀ (oral, rat) >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 2000 mg/kg |
| NIOSH | Not established |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10–50 mg |
| IDLH (Immediate danger) | NIOSH: Unknown |
| Related compounds | |
| Related compounds |
Pyrrolidine N-Methylpyrrolidine 2-Acetylpyrrolidine 4-Formylpyrrolidine 4-Hydroxy-pyrrolidine 1-Acetyl-pyrrolidine |