4,7-Dichlorobenzothiophene didn’t appear overnight but took shape through decades of organic chemistry research. Early in the 20th century, researchers aimed to expand the catalogue of benzothiophene derivatives, focusing on ring-substituted compounds. Through trial, error, and a strong curiosity for sulfur-containing heterocycles, they started to understand the structure–function relationships in chlorinated derivatives. As DDT and other halogenated aromatics hit the market, scientists paid more attention to the environmental and biological impact of these chemicals, and 4,7-dichlorobenzothiophene drew interest owing to its unique chlorine substitutions. Every chemist who worked with polychlorinated aromatic compounds contributed to the slow build-up of knowledge, eventually leading to efficient synthesis and detailed reports on this molecule.
The story of 4,7-dichlorobenzothiophene is tied to industrial processes where a sturdy, reactive aromatic backbone holds importance. With two chlorine atoms sitting at the 4 and 7 positions on a benzothiophene core, the compound offers more chemical versatility than its unsubstituted sibling. This molecule comes across as a pale crystalline solid, sometimes showing faint yellow hues, hinting at trace impurities or degradation products. Whether you’re opening a drum of this compound in a chemical processing plant or sketching out synthetic routes in a notebook, it’s hard not to notice the distinct, somewhat acrid odor common to many polychlorinated aromatic chemicals.
Measured as a solid under ambient conditions, 4,7-dichlorobenzothiophene features a melting point between 76 and 80°C, sometimes shifting depending on subtle variant impurities or batch conditions. It dissolves poorly in water and shows much more solubility in chlorinated solvents like dichloromethane. Chemical reactivity flows from the electron-withdrawing chlorines and the influence of sulfur in the bicyclic scaffold. This design brings stability toward oxidation compared to non-chlorinated versions, but it also suits the compound for electrophilic aromatic substitution under the right lab setup. Handling this blend of hydrophobicity, reactivity, and relatively high melting point calls for thoughtful lab technique.
Packing and labeling of 4,7-dichlorobenzothiophene put safety and clarity up front. Labels must list the IUPAC name: 4,7-dichloro-2,1-benzothiophene, followed by batch number, lot analysis (stating purity, usually above 97%), and recommended storage conditions (tightly closed containers, cool, dry locations away from light). Every shipment that I’ve seen arrives with a detailed COA (Certificate of Analysis) stating melting point range, GC or HPLC purity, and moisture level. In the research environment, analysts often run their own NMR and MS traces to double-check the supplier specs. UN numbers, risk phrases, and standardized hazard pictograms round out the necessary label information to keep everyone on the same page.
Synthetic routes began traditionally from benzothiophene, utilizing directed ortho-lithiation methods or halogen exchange techniques, but industrial practice favors direct chlorination of benzothiophene in the presence of Lewis acid catalysts. Chlorine gas and ferric chloride or aluminum chloride as the catalyst, applied under controlled temperature and atmospheric conditions, allows selective placement of the chlorine atoms. The process demands relentless monitoring: excess heat or prolonged exposure yields over-chlorinated byproducts. I’ve watched skilled technicians finesse the timing and quench steps to nudge the reaction mix away from tri- or tetrachloro derivatives. Some more recent literature shows phase-transfer catalysis helping scale-up and reduce waste, but regulatory and environmental pressures keep tightening around chlorination plants, so greener alternatives remain under exploration.
This compound’s main draw centers on its capacity for further functionalization. The chlorines at the 4 and 7 positions make standard nucleophilic aromatic substitutions possible: think reaction with amines, thiols, or alkoxides under the right heating or catalytic conditions. Suzuki and Stille couplings take advantage of haloarene reactivity, letting chemists bolt on extra aromatic groups or heterocycles, expanding application into pharmaceuticals and materials science. Modifications targeting the thiophene ring can introduce new sulfur oxidation states, but those often take more forcing conditions. Rarely does a benzylic ring escape such attention, making this scaffold a reliable starting point for future diversified synthesis rather than an endpoint.
Those who browse chemical suppliers frequently see 4,7-dichlorobenzothiophene labeled under a variety of handles. Synonyms include 4,7-DCBT, 4,7-dichloro-2,1-benzothiophene, and CAS Number 79501-15-8. Vendors might use catalogue codes like 4,7-DCBT or some numeric system. This variety hints at wider use or research presence, but can occasionally trip up folks searching regulatory or analytical databases.
No story about halogenated aromatics escapes without hard talk about safety. 4,7-dichlorobenzothiophene, like its relatives, can irritate skin, eyes, and mucous membranes. Chronic exposure presents a murkier picture, since animal toxicity data don’t always line up neatly with human effects. Lab workers preparing or transferring the material keep fume hoods running and don gloves, goggles, and lab coats. At scale, chemical plants build multiple layers of containment — spill trays, negative room pressure, extensive waste handling protocols — to prevent release into the wider environment. OSHA and REACH rules underline record-keeping and accident preparedness. A few times, I’ve seen near-misses with ruptured seals or leaking drums, always serving as reminders that physical safeguards need unwavering attention.
Most uses for 4,7-dichlorobenzothiophene center on acting as an intermediate in drug and agrochemical synthesis, along with specialty materials. Its structure fits designs for new antifungal, antibacterial, or anticancer compounds, as well as conjugated polymers for organic electronics. Structural chemists hunting for building blocks that balance reactivity with stability pick these kinds of chlorinated rings often, allowing modular assembly without losing grip on the sulfur core. In real-world settings, its modest air and light stability adds convenience to handling, but the niche applications arise because chemists keep seeking ring systems that make sense in advanced material and medicinal settings rather than commodity products.
Development teams within pharmaceutical and material science fields often hunt for new transformations of this molecule. Cross-coupling methodologies keep evolving, with milder catalysts and solvent systems that cut environmental footprint. Teams characterizing new derivatives keep turning up fresh biological activity — from kinase inhibition to antifungal or antibacterial properties — pushing research forward with promising leads. Technological advances such as continuous flow chemistry and machine learning predictions open up new possibilities. Machine-assisted retrosynthesis often highlights benzothiophene derivatives like this for their accessible functionalization, bridging computational and experimental processes for streamlined discovery workflows.
Although the exact human toxicity profile of 4,7-dichlorobenzothiophene has not reached the depth of some better-known halogenated aromatics, researchers devote significant time to animal models and in vitro studies to fill this gap. Reports point to moderate acute toxicity, with effects depending on exposure route and duration. A few chronic studies show liver and kidney stress markers in rodents at higher doses. Environmental chemists keep an eye on its persistence and behavior in soil or water, especially given historical concerns over polychlorinated compounds. The legacy of benzothiophenes in persistent organic pollutant research keeps pressure on labs and firms to track waste streams and handle effluent responsibly.
Looking ahead, the place for 4,7-dichlorobenzothiophene seems set to grow, as demand in specialty chemical synthesis and advanced materials ramps up. The need for greener synthetic approaches drives academic and industrial labs to test milder, less hazardous chlorination techniques or fully reengineered synthetic routes that skip direct halogenation. The broader push for sustainable chemistry means companies that invest in solvent recovery and closed system processes likely come out ahead. In the field, ongoing research into bioactivity hints at eventual appearance in next-generation pharmaceuticals or high-value devices, though only detailed risk-benefit studies and fresh regulatory reviews will chart the ultimate direction. For now, chemists still find this chlorinated benzothiophene a valuable tool on the lab bench, but future development depends on solving persistent safety and environmental concerns as much as unlocking new chemical potential.
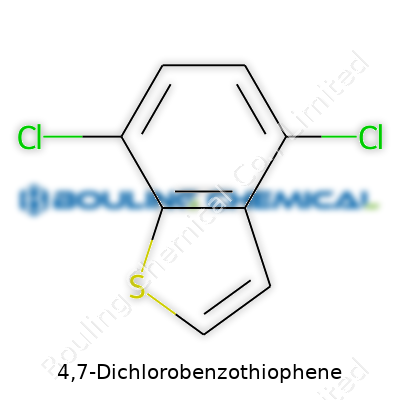
People who spend time in a lab or read through chemical databases often bump into names like 4,7-Dichlorobenzothiophene. The name sounds like something you’d want to keep locked away, but to chemists, it just describes a molecule with two chlorine atoms stuck onto a benzothiophene ring, specifically at the 4 and 7 positions. Picture a bicyclic structure – benzene fused to thiophene, and slide those chlorines right onto the ring. Its chemical formula: C8H4Cl2S. You end up with eight carbons, four hydrogens, two chlorines, and a single sulfur atom. Simple math, but the impact of this structure isn’t just an academic matter.
For a long time, folks outside the chemical or environmental industry might think a compound like this sits on a dusty shelf. In reality, 4,7-Dichlorobenzothiophene, like many similar molecules, pops up in conversations about pharmaceuticals, industrial byproducts, and environmental contaminants. This family of compounds has a way of linking science, safety, and health in a pretty tight knot.
In the chemical industry, folks use benzothiophene derivatives much like mechanics use a good wrench: they show up in the process of making dyes, medicines, and sometimes in the world of agricultural chemicals. Sticking chlorines onto the ring changes the behavior of the molecule, making it more stable in some reactions or, in some cases, making it linger longer in the soil or water.
With chlorinated hydrocarbons, concern isn’t just for folks working in chemistry. Persistent compounds like 4,7-Dichlorobenzothiophene can leave traces in rivers and soil, especially if they slip out from factories or landfill runoff. I remember reading a field report about how even low levels of these chemicals in sediment end up in the smallest critters, and just work their way up the food chain. Eventually, it lands in places nobody wants to see it – from fish in the river to milk on the kitchen table.
Keeping tabs on chemicals with formulas like C8H4Cl2S involves more than memorizing names. It asks us to decide where we’re willing to draw the line between industrial progress and the environment outside our window. The World Health Organization and the EPA have pressed for controls on chlorinated compounds because they can hang around far longer than anyone expects, and their long-term health effects remain hard to wipe away.
On a personal level, handling and disposing of chemicals needs care. It’s not enough just to file away the Material Safety Data Sheet and hope for the best. Better monitoring in factories — and more transparency about what actually gets released — means we don’t have to gamble with what’s in our water or food. In my time working with research teams, being up front about chemical inventory and spill response actually made cleanups easier, not harder. Responsible companies already track and reduce their usage.
Better testing at the local level gives communities more control and knowledge. Mandating regular soil and water testing near heavy industries isn’t overkill — it’s common sense if we want to head off bigger problems before they hit home. Seeing a formula like C8H4Cl2S may not light up headlines, but the impact from ignoring these compounds sure can.
Take a closer look at what goes on behind the scenes in crop protection, pharmaceuticals, and advanced materials, and 4,7-dichlorobenzothiophene keeps popping up. In the world of fine chemicals, it’s not just another building block. Chemists look at a compound like this the way a skilled chef eyes up a specific spice — a way to add complexity, depth, or an essential function to the final creation. Over the years, I’ve seen research teams turn to this molecule for one key reason: its structure gives them a reliable starting point for new molecules that can do some heavy lifting, especially in drug discovery and development.
Farmers need solutions that work on pests without hammering yields or harming the land. Modern crop protection chemicals rely on intricate molecules, and 4,7-dichlorobenzothiophene brings the right mix of stability and reactivity. I’ve talked with agronomists who say compounds based on this backbone help improve the safety and effectiveness of fungicides and herbicides. These tools shape the outcomes in fields and orchards across the globe. Of course, that also means attention needs to stay on safety, since the byproducts and residue can affect soil health and water supplies.
In the pharmaceutical industry, you won’t see this molecule on a bottle at the drugstore, but its fingerprint is there in the lab. Medicinal chemists hunt for core structures that can change how a drug behaves — how it gets absorbed, where it travels through the body, or how it binds. Adding chlorine atoms to a heterocycle, like you have here, can change the way drugs stand up to metabolism or how selective they are in hitting a target. I’ve watched teams chase tiny adjustments to molecules, sometimes swapping a single atom, trying to hit that sweet spot where a compound doesn’t just show up in tests but brings real clinical benefit.
Step out of the lab and onto the latest technology, and you find these kinds of molecules doing double duty in electronics. The presence of the thiophene ring — essentially a sulfur-rich backbone — makes it valuable in building organic semiconductors. As demand grows for flexible screens and lightweight solar panels, chemists use derivatives of benzothiophene for thin films and organic light-emitting diodes (OLEDs). The addition of chlorine atoms can fine-tune properties such as conductivity and stability. People sometimes overlook just how much of modern life leans on new materials science, but every device on my desk rests on molecules like these, tailored to do a job nothing else will.
No progress comes without hurdles. Synthetic pathways for making 4,7-dichlorobenzothiophene can require steps that generate waste byproducts, and that means chemical plants must pay attention to process safety and air emissions. Over the years, I’ve seen pushback from environmental watchdogs after accidents or cleanups along riverbanks. Improvements are coming, with researchers searching for greener methods, maybe by recycling reagents or cutting energy use with better catalysts.
Safe handling and proper training are non-negotiable. In my own experience, companies that treat chemical safety as a culture rather than a checklist see fewer accidents and respond better to the unexpected. Investing there not only protects workers but cuts long-term costs.
Public demand keeps growing for solutions that don’t just work, but also respect the environment. Continued pressure from regulators, and a willingness in the industry to innovate, could turn the story of 4,7-dichlorobenzothiophene into a case study for safer, cleaner chemistry.
Stepping into the world of specialty chemicals, even a small shift in purity can throw off an entire synthesis route. Take 4,7-Dichlorobenzothiophene—a compound that hovers over many research benches, thanks to its role in pharmaceuticals, advanced materials, and even organic electronics. Purity here isn’t just a marketing buzzword; it draws the line between progress and setback. By my own experience scraping out yields for weeks on a project, the difference between a 97% sample and 99% could make or break the goal product.
Chemical catalogs and suppliers often land on a minimum purity specification of 98% for 4,7-Dichlorobenzothiophene. Going by Sigma-Aldrich and TCI, that figure offers a good trade-off: fewer headaches from side products and a smoother time downstream. Chasing higher purity—up to 99% or above—serves those who can’t afford interference, like semiconductor developers or pharmaceutical chemists where dusting the surface with another impurity risks a failed lot.
An honest confession: It’s tempting to grab whatever’s available under a deadline, thinking a percent or two won’t make a difference. Then, the NMR looks like modern art, columns run like molasses, and the cycle of trouble begins. On paper, a tighter purity cut won’t magically transform chemical success, but it prevents that tedious detective work down the line. The cost does go up, but for research or high-value manufacturing, the costs of letting impurities roam free quickly outweigh that up-front saving.
The stakes rise because benzothiophenes attract stubborn contaminants. Isomers, byproducts, unreacted starting materials, and moisture all creep in. Failing to account for these impurities can wreck spectral clarity or poison a catalyst in the next reaction, turning what should be a straight path to a product into an expensive maze. I’ve run into situation after situation where that last stubborn impurity sneaks past and skews the whole experiment—something you only notice late, when the puzzle pieces don’t fit together cleanly.
Chemical producers rely on a lineup of tests—HPLC, melting point checks, and GC-MS—to keep the specification honest. Labs willing to double-check every bottle with a quick NMR save themselves a mess of repair work. Solid documentation—certificates of analysis showing those chromatograms, supported by spectral data—offers buyers more than a number on a spreadsheet. I’ve learned to ask for the data, not just the claim, before putting any new supplier’s word to the test. That small step brings plenty of peace of mind, knowing exactly what goes into a project, not just hoping for the best.
Extra care with specifications saves later regrets. Labs cranking out routine screens often skip the highest grades, fixing problems as they pop up. But if the outcome hangs on every atom doing its job, squeezing out that last point of purity pays dividends. Communication across teams also matters—letting everyone know what minimum purity truly supports the workflow, not blindly following catalog choices or price points. Picking partners with solid reputations, inspecting their track record for quality, and holding them accountable through documentation turns procurement from a dice roll into a calculated decision.
As much as chemistry lauds innovation, the basics—knowing what is in the bottle, not just on the label—build better structures, syntheses, and results. From experience, investing in the right purity level for 4,7-Dichlorobenzothiophene is no luxury; it’s simply smart science.
A lot of folks talk about chemical storage like it's just a checklist. That's not how things work in the real world, especially with compounds like 4,7-Dichlorobenzothiophene. This isn’t some household cleaner you can leave under the sink. Improper handling leads to serious risks—spills, contamination, or even long-term health headaches. I’ve been around labs long enough to see what happens when corners get cut. Most mishaps start with the tiniest oversight in storage.
4,7-Dichlorobenzothiophene belongs to a class of compounds known for giving off toxic fumes if mishandled. People who have worked with similar chemicals know that even opening a container in the wrong spot is enough to trigger a whole-room evacuation. Its reactivity and volatility mean ordinary cabinets don’t cut it. Once, I saw someone stack it near an old bottle of acid—it didn’t take long for everyone to start smelling something “off.” That should never happen.
A secure, dedicated space trumps convenience every time. If you ask anyone who’s managed a safe chemical storeroom, they’ll probably tell you about the value of segregation. 4,7-Dichlorobenzothiophene goes in airtight containers, properly labeled, and far from anything reactive, especially acids or oxidizers. A fume hood nearby isn’t just nice to have. It’s a real line of defense against accidental exposure. Glass or high-density polyethylene containers handle the job—no metal lids or mystery jars.
Temperature swings spell trouble for chemicals like this. Store it cool and dry, away from sunlight or any source of heat. Someone once stuck a batch near a window “just for a few days.” That ended with crusty, degraded material and a nasty stench. No one wants a cloud of mysterious vapor in their face. Constant ventilation keeps the air fresh and prevents any accidental buildup of gases. Relying on a closet fan doesn’t work; go for real chemical ventilation systems that pull fumes outside and away from people.
Labeling isn’t just a box-ticking exercise. Clear hazard warnings serve as reminders every time someone opens the cabinet. Spill control materials should stay close at hand. Absorbent pads, neutralizing agents, and thick gloves need to be part of the setup, not an afterthought. Remembering the right personal protective equipment matters—a set of goggles and a solid pair of gloves becomes your best team once something spills or leaks.
I’ve seen teams get complacent after months without problems. That’s risky thinking. Regular checkups, inventory tracking, and spill drills turn safe storage from theory into practice. If you don’t remember the last time you reviewed storage procedures, then it’s time to put it on the calendar. Even quick refreshers help cut down on mistakes nobody wants to deal with.
Manufacturers and research groups can team up to design packaging that’s both rugged and easy to track. That keeps mistakes rare and makes audits less of a headache. Automated inventory might sound expensive, but it pays back with peace of mind. Facilities should push for digital logs and video monitoring; those extra layers make it easy to spot a missing bottle before anything bad happens. In the end, it’s about putting people and safety before convenience—each step, each label, each locked cabinet.
Handling chemicals tends to come with a certain routine, but 4,7-Dichlorobenzothiophene isn't your ordinary substance. It packs a toxic punch that can throw off even those who treat lab work as second nature. I remember once grabbing a bottle of dichlorobenzothiophene with nothing but thin latex gloves—my hands started to sting after just a minute. Turns out, this stuff can slip through skin protection much faster than expected.
This compound often acts as an intermediate for making drugs or pesticides, but it also produces fumes that irritate the eyes, nose, throat, and lungs. On top of that, skin contact can lead to redness, rashes, sometimes even long-term sensitivity. Swallowing it? Even tiny amounts will ruin your day, with headaches, nausea, or worse.
Lab coats and splash-proof goggles become your best friends with compounds like this. Use thicker nitrile gloves rather than standard latex. If you run short on safety goggles or good gloves, put off the work until you find them. I've seen some try to tough it out—they’re the first to end up in the emergency room for chemical burns or breathing trouble.
Forget open-air benches; fume hoods or well-designed local exhaust systems make all the difference. Once, we tried to skip the fume hood to speed up a reaction, thinking the room’s air filters would manage. We all ended up coughing within minutes. Vapor builds up fast, and ordinary airflow can’t keep up.
Contain spills by working over trays and storing extra absorbent pads nearby. Trying to clean up a spill on bare skin will just spread contamination—use proper tongs and disposables. Toss everything that soaks up the chemical in a sealed, labeled hazardous waste bin. That might seem wasteful, but you don’t want extra “souvenirs” sticking around on a shared bench.
If eyes get a splash, wash with an eyewash station right away. I once hesitated for only a minute after a splash, thinking it’d clear up, but the burning got worse. A simple fifteen-second delay can mean days of discomfort. For skin contact, scrub with soap and lots of water; with inhalation, get out of the room. Head straight to a healthcare provider if symptoms crop up—don’t wait.
Store the chemical in a locked cabinet built for volatile organics. Label containers with bright, unmistakable warnings. Once, a bottle migrated onto an open shelf near the snacks by mistake—nobody touched it until a strong chemical smell filled the breakroom.
Read the safety data sheet every single time you open a new bottle, even if you’ve handled it before. Join regular safety training—and don’t skip the incident reviews that some labs hold after a mishap. That’s where most folks learn what shortcuts cause real problems.
Workplaces can do more by investing in better safety gear and updating emergency procedures. At my old job, just switching to color-coded warning labels kept several accidents from happening. Open conversations about near-misses help, too, because telling stories turns scattered rules into habits people actually remember.
| Names | |
| Preferred IUPAC name | 4,7-dichloro-1-benzothiophene |
| Other names |
4,7-Dichloro-1-benzothiophene Benzothiophene, 4,7-dichloro- 4,7-Dichlorobenzo[b]thiophene |
| Pronunciation | /ˈfɔːr ˈsɛvə(n) daɪˌklɔːr.oʊˌbɛnˈzəʊˌθaɪ.oʊˌfiːn/ |
| Identifiers | |
| CAS Number | [20856-16-8] |
| Beilstein Reference | 136872 |
| ChEBI | CHEBI:89709 |
| ChEMBL | CHEMBL3376877 |
| ChemSpider | 16736605 |
| DrugBank | DB07855 |
| ECHA InfoCard | 100.011.713 |
| EC Number | 208-725-4 |
| Gmelin Reference | 153661 |
| KEGG | C19201 |
| MeSH | D017937 |
| PubChem CID | 121364 |
| RTECS number | GK8750000 |
| UNII | 1121P760S3 |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C8H4Cl2S |
| Molar mass | 241.14 g/mol |
| Appearance | White to light yellow powder |
| Odor | Odorless |
| Density | 1.54 g/cm³ |
| Solubility in water | Insoluble |
| log P | 3.9 |
| Vapor pressure | 0.00156 mmHg at 25°C |
| Acidity (pKa) | 3.84 |
| Magnetic susceptibility (χ) | -82.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.6680 |
| Viscosity | 1.31 cP (25°C) |
| Dipole moment | 2.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 339.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | 4,7-Dichlorobenzothiophene: 89.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4777.8 kJ/mol |
| Pharmacology | |
| ATC code | '' |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation, toxic to aquatic life with long lasting effects |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P362+P364, P333+P313, P337+P313, P501 |
| Flash point | Flash point: 146°C |
| Autoignition temperature | 250 °C |
| NIOSH | SN8751000 |
| PEL (Permissible) | PEL (Permissible): Not established |
| Related compounds | |
| Related compounds |
Dibenzothiophene Benzothiophene 4-Chlorobenzothiophene 7-Chlorobenzothiophene 4,7-Dibromobenzothiophene 3-Chlorobenzothiophene 4,7-Dimethylbenzothiophene |