A look into the evolution of 4-(6-Methylbenzothiazol-2-Yl)Aniline reveals how curiosity and practical demand have driven discoveries in aromatic chemistry. Early progress around benzothiazole derivatives rolled out during the last century as dye makers scoured for compounds with better colorfastness. The methyl group at the 6-position of the benzothiazole ring, coupled with an aniline at the 4-position, emerged from focused chemical research aimed at tuning electronic profiles and reactivity. Real breakthroughs came as analytical equipment sharpened—users could spot not only structure but also how small changes empowered broader applications, especially in electronics and life sciences. Long stretches of trial-and-error marked the search for better intermediates, with technicians coaxing out new syntheses and shifting production from small flasks to pilot-scale reactors. Not just a journey of numbers, this chemical’s story includes persistent people, midnight runs in the lab, and the slow tightening of safety protocols as understanding grew.
As a distinct aromatic amine, 4-(6-Methylbenzothiazol-2-Yl)Aniline grew popular for its adaptability across different sectors. Chemists turned to this molecule to push boundaries for new fluorescent dyes, photochemical sensors, and sometimes organic semiconductors. Its unique arrangement of nitrogen and sulfur atoms in the benzothiazole core brings specific optical and bonding properties, letting the compound serve as a flexible building block. What attracts ongoing attention is its role in further synthesis, where it behaves reliably—no unexpected surprises during coupling, no sidetracks pinching reaction yields. It doesn’t stand alone for glory but regularly shows up in high-value recipes, earning respect among those who value consistent performance over hype.
With an appearance that tends toward pale yellow to brown solid, 4-(6-Methylbenzothiazol-2-Yl)Aniline brings a keen balance between rigidity and meltability. Typical melting points rest in the range of 150–160°C, making it manageable in glassware and industrial setups without worrying about runaway heating. You get a moderate solubility in polar organic solvents—think acetone, DMSO, or even chloroform—while water resists dissolving much of it. The methyl group tweaks the electron density, nudging its behavior a step away from other benzothiazole or aniline cousins. These properties set the stage for how it fits into chemical reactions, especially where selectivity and stability matter. I recall curious students watching thin-layer chromatography plates light up under ultraviolet, noting the compound’s strong fluorescence and piecing together why such behavior could be useful in sensor design.
Industry standards call for high-purity grades, often above 98%, to avoid gumming up complicated syntheses downstream. I’ve seen labs and suppliers hash out specs like single-point melting ranges, color indices, residual solvents, and HPLC retention times. Labels spell out hazards calmly: avoid inhalation, minimize skin contact, store cool and dry, and never mix leftovers with incompatible acids or oxidizers. Regulatory bodies in Europe and North America have demanded clear pictograms, batch numbers, and traceable origins after a few sad episodes of cross-contamination or mistaken IDs in the past. These tools help users stay informed and safe, rooting out ambiguity before it has a chance to create a mess in the lab or workplace.
Most reliable syntheses start with 6-methylbenzothiazole and introduce aniline counterparts through nucleophilic aromatic substitution or amination. Acid catalysis or copper-mediated coupling remains a workhorse in many routes, balancing reasonable cost with strong yields. Lab workers have worked out protocols to streamline purification—crystallization from ethanol, followed by careful vacuum drying to squeeze out remaining solvent traces. Over the years, greener methods popped up; microwave-assisted reactions and solvent minimization have begun to replace older routines where ten liters of hot DMF circulated for hours. Watching the chemistry world respond to both economic and environmental concerns reassures me that progress is a communal effort, driven by local improvements shared in international journals and at late-night conference roundtables.
This compound’s backbone allows for several creative modifications, especially in the world of dye chemistry and pharmaceuticals. Simple N-alkylation or acylation at the aniline nitrogen tailors solubility. Electrophilic substitutions at the aromatic rings punch up reactivity for more specialized uses in material science. I’ve seen the benzothiazole nucleus serve as a launchpad for sulfonation and halogenation, which can dial in new photophysical properties. In cross-coupling reactions (like Suzuki or Buchwald-Hartwig), the structure holds up well, opening doors to even bigger molecules. Researchers tracking quantum yields and electron transfer rates always circle back to the importance of ring substitutions—the methyl and aniline play equal parts in making novel compounds possible while holding stability steady.
Depending on catalog or supplier, this molecule goes by names such as 4-(6-Methyl-2-benzothiazolyl)aniline, 4-Anilino-6-methylbenzothiazole, or BTZ-4MA. Some technical documentation might shorten it to MBT-Aniline or 6-Me-BZT-aniline, especially in research circles juggling many candidates. Such naming differences sometimes throw off new students, but the structure stands as a signature fingerprint for anyone with a decent chemistry background. Standard identifiers include CAS numbers, registry data, and sometimes internal codes for private-label offerings at specialty chemical firms.
Lab handling demands respect and routine. Though not classified as acutely toxic, 4-(6-Methylbenzothiazol-2-Yl)Aniline poses hazards through prolonged skin or eye contact, and its fine powders can irritate airways if mishandled. Gloves, goggles, and solid local ventilation serve as my frontline tools—a knock-over or an open vial during weighing reminds any practitioner how quickly dust can travel. I saw one incident where improper disposal led to unexpected reactivity in mixed waste, underscoring the wisdom of correct segregation and record keeping. International guidelines (like GHS, REACH, and OSHA) have tightened best practices, so most academic or industrial setups now include routine monitoring, detailed MSDS posting, team drills, and emergency showers. Over the years, better packaging and updated risk analyses sharply reduced accidental exposure rates.
The value of this compound shows up in a range of real-world uses that I’ve seen expand year by year. Dye and pigment development always benefits from versatile aromatics such as this, especially where durability against sunlight and repeated washing crosses with custom colors. Researchers designing organic light-emitting diodes (OLEDs) and solar cells gravitate toward benzothiazole derivatives for their charge-transport features. Sensing technology leverages the molecule’s fluorescence; here, chemical sensors for heavy metals or biological markers require stability, selectivity, and visibility—all packed into this rigid yet modifiable frame. Pharmaceutical chemists explore the structure as a starting point for antimicrobials or central nervous system drugs thanks to the distinctive electronic environment surrounding both the aniline and thiazole rings. In every branch, robust test results and peer-reviewed studies point to growing demand, with both open-source and proprietary methods shaping the pace of progress.
Most recent R&D pushes investigate new derivatives and functionalizations, shining a light on both opportunities and stubborn bottlenecks. Teams often pair computer modeling with hands-on synthesis, cutting down unnecessary experiments and zeroing in on higher yields or new activities. Collaborative projects across Asia, Europe, and the Americas exchange ideas for improving stability under operational stress or for merging this scaffold with other heterocycles. Pioneering labs sometimes experiment with thin film fabrication or inclusion within composite materials for electronics. In spectroscopy and analytical circles, the search continues for intense, reproducible fluorescence to unlock sharper detection tools for environmental and biomedical monitoring. I’ve seen focused grant funding raise the bar for sustainable syntheses and for understanding long-term breakdown products—topics that often straddle borders between academic curiosity and pressing regulatory need.
While 4-(6-Methylbenzothiazol-2-Yl)Aniline hasn’t been flagged as highly hazardous, studies still carve out the potential risks to both people and ecosystems. Chronic exposure investigations map out the compound’s metabolic fate—testing whether breakdown products could bioaccumulate or linger in soil and water. Lab-based mutagenicity, cytotoxicity, and acute exposure studies provide a baseline, but emerging data encourages caution with higher concentrations or prolonged work. Safety data sheets spell out careful cleanup and quick spill response, all shaped by evolving understanding from both animal models and cell studies. This approach—treat every unknown with due respect—balances progress against responsibility, nudging chemists to keep up with literature and adapt protocols as knowledge advances.
Looking ahead, 4-(6-Methylbenzothiazol-2-Yl)Aniline will likely feature in more targeted and multidisciplinary research, all thanks to its reliable structure, tunable properties, and broad compatibility with other building blocks. Big opportunities loom in organic electronics, medical imaging, and green chemistry, where demand grows for stable yet biocompatible materials. I’ve noticed increased interest in low-impact synthesis methods, including flow chemistry and catalyst recycling. Regulatory hooks around environmental impact and worker safety drive innovation, pushing suppliers and end-users to share data and refine methods collectively. As next-generation sensors, display materials, or drugs arise from these research efforts, this compound’s place stays secure—a testament to both its solid performance and the community’s ability to reinvent how classic molecules find modern use.
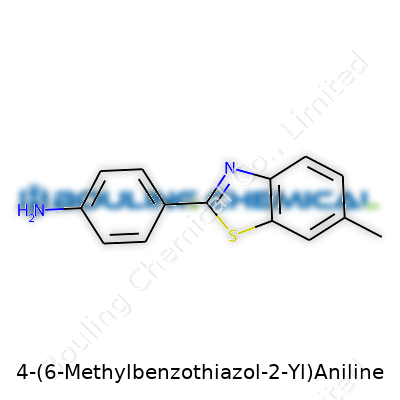
No matter how complex a name might sound, every molecule has a story—4-(6-Methylbenzothiazol-2-Yl)Aniline tells its own through atoms and bonds. At its core, this compound brings together two recognizable organic structures: benzothiazole and aniline. The aniline part comes with a benzene ring attached to an amino group. That just means there’s a cluster of six carbon atoms set in a ring, each paired with hydrogen, and one carbon hosts a nitrogen-bearing -NH2 group. Now, stick that at the fourth position; you start to see the scaffolding of something more than a textbook example.
The real twist comes from the other side: the 6-methylbenzothiazole unit connects at the benzene ring’s fourth point. Benzothiazole, as old lab work reveals, has both a nitrogen and a sulfur atom built into another six-sided ring. A methyl group hangs off the sixth position, tipping the balance, and giving the structure extra character. Years of running melting point tests show that a methyl group can make a molecule more volatile than expected, and sometimes easier to work with in solution. The two rings, fused by the nitrogen and sulfur, carry unique electronic properties. These features make the molecule attractive for folks interested in dyes, drugs, or chemical sensors.
Chemists chase after molecules like this because adding groups such as methyl or amino shift the way the electrons flow inside a molecule. My time in pharmaceutical research showed how these tweaks can change how a molecule binds to a protein or moves through the body. For example, the benzothiazole segment is known for its biological activities, popping up in antibacterial and antifungal trials. Aniline brings its own history as a key base for many dyes and pigments. Put these together, and you get possibilities for new drugs, new sensors, or even advanced materials with special color-shifting abilities.
From my bench-top synthesis days, I know the layout of a molecule rules the day. Arrange atoms just so, and you light up new uses. Miss a single group, and the properties vanish. In 4-(6-Methylbenzothiazol-2-Yl)Aniline, the methyl group at the sixth spot isn’t only for show—it can block or direct chemical reactions, and it’s useful in tuning how the whole molecule behaves in living systems. The fused rings share electrons in ways that plain benzene can’t match.
This structure points a way forward for chemists seeking tailor-made functions. Swap out the methyl for something bigger, or change the location of the aniline group, and results shift—sometimes dramatically. In real-world labs, those little changes decide between a useful diagnostic and a failed project. Safety always stays top-of-mind, since aromatic amines make regulators tighten rules, so scientists have to balance innovation with responsibility.
Understanding this chemical’s structure goes far beyond memorizing atom placements. It’s about seeing how shape, size, and small modifications change everything—from color to medical potential. The molecule isn’t just ink on paper; it translates into real jobs and real products in laboratories, factories, and hospitals. By knowing why the pieces fit together, future scientists and innovators can push boundaries, designing safer, smarter, and more effective compounds for the next challenge.
You come across 4-(6-Methylbenzothiazol-2-Yl)Aniline in specialty chemical catalogs, but this isn’t just another tongue-twister name in organic chemistry. Researchers working in dye chemistry know this molecule well. Its structure, based on both benzothiazole and aniline groups, lets it deliver intense color and reliable stability. Textile labs put it to work in disperse dyes—these dyes color polyester and synthetic fibers, which rarely absorb other compounds evenly. After years in material science, I’ve seen how the unique arrangement of this compound translates into vibrant clothing that keeps its color, even after dozens of wash cycles.
Laboratories rely on molecular probes to trace chemical reactions or spot specific diseases. 4-(6-Methylbenzothiazol-2-Yl)Aniline often appears in fluorescence research. By tweaking the side groups, chemists generate bright signals that reveal the presence of metal ions or biomolecules. For example, a research team in Germany demonstrated how a benzothiazole-based aniline could light up mercury in polluted water samples. In medical diagnostics, fluorescence imaging built on molecules like this one can help doctors pinpoint tiny tumors earlier than standard scans permit.
Watch displays and organic LEDs have changed screens over the past two decades. Behind these advances, organic molecules form the backbone of luminescent layers. Devices need organic semiconductors that blend flexibility, color purity, and a long operational life. Engineers testing materials in display prototypes often use benzothiazole-aniline derivatives. The methyl group anchored in this molecule steers its electrical characteristics, enabling smooth charge transfer. As OLED demand grows, the search for compounds that balance brightness and lifespan keeps bringing scientists back to well-studied scaffolds like this one.
Sunlight wreaks havoc on materials over time. Ultraviolet stabilizers slow fading in plastics, paints, and even skincare creams. The benzothiazole core linked to aniline helps shield materials from breakdown caused by UV rays. In some UV-absorbing agents used in coatings, chemists pick structures related to 4-(6-Methylbenzothiazol-2-Yl)Aniline because they absorb the damaging part of the spectrum and scatter the energy harmlessly. The right stabilizer cuts down on microplastic production and pigment loss in outdoor furniture, keeping these goods looking fresh longer.
As with many specialty chemicals, toxicity and environmental persistence spark concern. During my time working with regulatory teams, the best safeguard always came from up-to-date toxicology data and clear communication with suppliers. Scientists keep engineering greener variants, aiming for better degradation in waste streams. The move to transparent labeling and international safety sheets also helps industrial end-users select products responsibly. Teams designing next-generation fluorescent tags or UV stabilizers look for lower-hazard substitutes, but acknowledge that replacing a well-characterized molecule calls for meaningful investment in research.
Experience in material science points to a future where molecules like 4-(6-Methylbenzothiazol-2-Yl)Aniline serve as stepping stones, not end points. Collaboration between universities, industry, and regulators can ensure both continued innovation and responsible use. If new applications arise, trust in data-driven decisions and open dialogue remains key. The journey of this compound illustrates how chemistry underpins technologies that touch every part of daily life—sometimes with colors or brilliance people take for granted.
Chemicals like 4-(6-Methylbenzothiazol-2-yl)aniline don’t ask for much, but they don’t forgive carelessness either. I remember my early days in the lab. Leaving anything out, even overnight, meant risking unexpected reactions or ruined experiments. This compound, used often in analytical labs and research, shows its best side only when you give it the respect proper storage brings.
Think of most organic powders as delicate—heat nudges them, and sometimes the transformation isn’t visible, but purity drops. Storing this aniline derivative in a cool, dry place guards its structure. At the university, we didn’t have climate-controlled vaults, but room temperature, away from heat sources and sunlight, kept the bottle for months without problems. Store it in a closed cabinet, away from radiators, direct window light, or spots prone to humidity. If you ever notice caking or strange odors, it’s a sign the chemical has started to degrade.
Some folks shrug at the risk moisture brings. I've seen samples absorb water from the air in just a day—clumping, sometimes turning sticky. For 4-(6-methylbenzothiazol-2-yl)aniline, screw the lid on tight after each use. Silica gel packets in the storage cabinet do wonders. It doesn’t require nitrogen-flushed gloveboxes, just basic vigilance. Don’t let it sit in open air for more than you need to weigh or transfer it.
Even experienced chemists get lax, but gloves and safety glasses belong on every time the container opens. Dust or a stray particle from similar aromatic amines causes nasty irritation. I saw a colleague toss a little powder in the trash once, only for housekeeping to come by and get exposed—simple oversight, lasting consequences. Always transfer small amounts in a fume hood, wipe your bench, and label containers clearly.
It’s easy to treat storage as an afterthought, but the cost of a contaminated or decomposed standard sets projects back weeks. Reliable results come from pure materials, and pure materials come from mindful storage. Research shows some benzothiazole compounds react to light or degrade in high humidity, reducing their effectiveness in analytical applications. A dark bottle, sealed tight, in a dry cabinet, gives peace of mind.
If your workplace runs on good habits, these steps save you money and time:
Good storage reflects a good lab culture. Most problems I’ve seen over the years boiled down to someone ignoring that simple principle. Treat chemicals well, and they’ll perform as they're supposed to—there’s no shortcut around that.
Walking into a lab or a factory, you pick out labels almost subconsciously. Some names peppered on drums grab your attention immediately. 4-(6-Methylbenzothiazol-2-Yl)Aniline tends to do just the opposite. Few outside chemistry circles stop to wonder whether this mild-sounding compound deserves a closer look, but ignoring tricky chemicals often leads to headaches—sometimes literally.
Despite its tongue-twister name, this chemical doesn’t show up in consumer products on grocery store shelves. Folks in pigment manufacturing, plastics, or specialty dyes might see it more often. My days working in a small dye facility showed me the danger lies in things we know least about. I’ve watched experienced workers shrug at unfamiliar compounds until skin rashes or a sudden coughing fit calls for the safety data sheet.
With 4-(6-Methylbenzothiazol-2-Yl)Aniline, the conversation gets technical fast. The chemical structure, built off both benzothiazole and aniline, should trigger alarm bells. Benzothiazole rings feature in derivatives flagged for environmental and health issues. Aniline, the second half of the molecule, doesn’t play nicely either—its reputation stinks from its own toxic footprint. Combining the two in one molecule sets the table for uncertainty unless solid data says otherwise.
Go digging in public chemical hazard databases like PubChem, ECHA, or OSHA, and you don’t get a big flood of safety information for this particular compound. That’s never a sign to relax. Lack of data nearly always means the compound hasn’t had its time in the spotlight, not that it’s harmless. I learned from working in chemical safety: assume risk, not innocence, when the evidence is thin. Too many “unlisted” substances ended up with serious precautions down the line.
Known aniline-based chemicals can affect blood, causing methemoglobinemia—a nasty condition, where oxygen can’t do its job—all from inhaling dust or brushing unprotected skin. Benzothiazole-related compounds also ring alarm bells for aquatic toxicity. Considering the mix, nobody should treat 4-(6-Methylbenzothiazol-2-Yl)Aniline like ordinary kitchen salt.
Personal experience teaches that hazards don’t always come with fireworks. A chemical splashed on skin or left to build up as dust may not trigger a reaction instantly. Some risks, like allergenicity and long-term toxicity, take months or years to show up. Too many times, proper ventilation, gloves, and dedicated waste streams seemed “excessive”—until several workers developed contact dermatitis or persistent coughs. At that stage, corner-cutting turns into a story about hospital visits, not business-as-usual.
My standard playbook for compounds in the grey zone: keep the material contained, avoid generating dust or vapor, and never trust unlabeled containers or lines. Chemical goggles and gloves stand as the easiest shield against the unknown; regular training reinforces respect, not fear. Spills call for prompt clean-up with equipment on—not towels and a shrug. Smart companies hold suppliers to account for full material disclosures, insisting on updated safety data sheets with each delivery. If disposal instructions sound vague, assume the chemical can harm water or soil. In my experience, the extra cost of good waste treatment always beats the price of a lawsuit or environmental fine.
Ignoring questions about chemicals like 4-(6-Methylbenzothiazol-2-Yl)Aniline never worked out for anyone—not the workers, not the company, not the neighborhood. The path forward shines brightest where experience, facts, and a little bit of humility set the rules. With every unknown chemical, treating uncertainty as a call to caution means fewer regrets for everyone involved.
Chemicals only work as well as their purity. With 4-(6-Methylbenzothiazol-2-Yl)Aniline, lab buyers and production chemists want to see high-grade product. From published catalogs and supplier sites, this molecule gets offered mainly in purities upwards of 97%. This is not an accident. Small-scale researchers and industrial formulators both rely on every gram working as planned. Lower grades would leave too many unknowns—extra steps, wasted batches, unreliable reactions.
Lab people quickly spot the difference between a 95% and a 99% chemical. Picture a fluorescence study or a pharmaceutical screen. Even a hint of stray material can throw off a result. By now I’ve seen enough chemical analysis runs go off the rails to know purity is more than just a label. High-performance liquid chromatography (HPLC) and nuclear magnetic resonance (NMR) results don’t lie.
Technically, reputable vendors will include purity certificates or COAs for every batch. Read one from a trusted supplier, and you’ll often see levels well above the stated minimum—sometimes buyers spot product at 98% or better, even when 97% is guaranteed. Researchers often request more detail: moisture content, melting point, even traces of heavy metals. This kind of paperwork helps answer the bigger question of trust and supplier reliability.
You buy what fits the job. Academic researchers looking to run pilot reactions won’t shell out for kilo drums. Instead, you mostly see 4-(6-Methylbenzothiazol-2-Yl)Aniline show up in small vials—1 gram, 5 grams, maybe 10 grams if someone is scaling up a method. These sizes travel well and cut down on waste, which matters if a lab fridge is overflowing.
On the other hand, industry buyers who know exactly what they need head straight for 25-gram, 100-gram, or even half-kilo jars. Custom runs can go larger—sometimes 1 kilo or packaged per customer spec, but that takes a conversation with the sales team. Every container comes sealed, labeled, and often double-bagged inside. I’ve helped research groups sort through shattered bottles or contaminated powder before. A supplier who gets packaging right by using sturdy jars or blister-resistant packs becomes the default choice next time.
Shipping loss, spoilage, and cross-contamination cost more than the chemical itself. An extra outer carton or desiccant pack can save countless headaches. For regulated buyers—say, in pharma QA domains—tamper-evident closures often become non-negotiable.
Materials science moves fast today. Analytical-grade chemicals like this one have clear shelf lives with real performance data backing up every claim. Reports from Sigma-Aldrich, TCI, and others confirm purity levels through repeated testing. Regulators, including the FDA and EMA, set public expectations with guidelines about raw material traceability. Scientists and purchasing departments review SDS sheets and run batch-quality crosschecks because real experiments depend on these controls.
A reliable supply chain also matters for folks scaling up. Breaches of quality—unexpected solvent residues or mismatched molecular weights—can put a halt to months of work. Nobody wants to re-run toxicity tests just because one shipment slipped below spec.
People trust brands that deliver both quality and consistent packaging. Strong supplier relationships rest on transparency—batch test results, accurate labeling, and practical, sturdy containers. That’s what builds confidence in the chemistry and everyone using it.
| Names | |
| Preferred IUPAC name | 4-(6-Methyl-1,3-benzothiazol-2-yl)benzenamine |
| Other names |
4-(6-Methyl-2-benzothiazolyl)aniline 4-(6-Methylbenzo[d]thiazol-2-yl)aniline |
| Pronunciation | /ˈfɔːr sɪks ˈmɛθɪlˌbɛnzoʊˌθaɪəzɒl tuː aɪl əˈnɪliːn/ |
| Identifiers | |
| CAS Number | 654672-85-2 |
| 3D model (JSmol) | `4WZI` |
| Beilstein Reference | 3715891 |
| ChEBI | CHEBI:136543 |
| ChEMBL | CHEMBL2104567 |
| ChemSpider | 23815818 |
| DrugBank | DB08346 |
| ECHA InfoCard | 03d9fd10-024d-492d-b02c-3eeb9e6faf61 |
| EC Number | EC 686-204-4 |
| Gmelin Reference | Gmelin 833893 |
| KEGG | C41853577 |
| MeSH | C16H14N2S |
| PubChem CID | 118240617 |
| RTECS number | GV8600000 |
| UNII | PUP8I14G73 |
| UN number | Not classified |
| CompTox Dashboard (EPA) | DTXSID4060431 |
| Properties | |
| Chemical formula | C14H12N2S |
| Molar mass | 256.34 g/mol |
| Appearance | Light yellow to yellow powder |
| Odor | odourless |
| Density | 1.3 g/cm³ |
| Solubility in water | Insoluble |
| log P | 2.96 |
| Acidity (pKa) | Heterocyclic aromatic amine, pKa ≈ 4.3 |
| Basicity (pKb) | 11.03 |
| Refractive index (nD) | 1.761 |
| Dipole moment | 3.87 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 172.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4757 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Harmful in contact with skin. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | InChI=1S/C14H12N2S/c1-10-3-2-4-11-12(10)16-14(17-11)8-5-6-13(15)7-9-14/h2-9H,1H3 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P308+P313, P321, P333+P313, P362+P364, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-0-₩ |
| Flash point | > 242.1 °C |
| Lethal dose or concentration | LD50 oral (rat) > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 5000 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 250 mg |
| Related compounds | |
| Related compounds |
Benzothiazole 4-Aminobiphenyl 6-Methylbenzothiazole 4-(Benzothiazol-2-yl)aniline 2-(4-Aminophenyl)benzothiazole |