Chemists started looking at thiazole derivatives over a century ago, finding new rings with nitrogen and sulfur that opened doors for dyes and medicines. The introduction of a pyridyl group, especially at the 4-position, set the stage for compounds with tweaks that changed their electronic and biological behavior. Over time, researchers noticed 4-(4-Pyridyl)-3H-Thiazole-2-Thione stood apart—it showed promise in both laboratory and practical applications. After World War II, as researchers dug deeper into heterocycle chemistry, this compound found its way into synthetic strategies and caught the eyes of those studying structure-activity relationships. During the 1960s and 70s, efforts to improve how these heterocycles were made and used in pharmaceutical frameworks brought this molecule into focus for its unique balance of donor-acceptor properties.
4-(4-Pyridyl)-3H-Thiazole-2-Thione shows up as a solid with a faint yellowish or tan color, picked out of a reaction flask by chemists as both a research target and an intermediate. Many chemical supply catalogs list it for research and advanced synthesis. Behind its name is a promise: the thione group attached to a thiazole makes it possible to join with a wide range of substrates, and the pyridine ring brings further versatility. Academic labs keep this compound on hand for exploratory synthesis, catalysis, and as a starting material for next-generation research. Some teams in pharmaceutical development use it to build more complex systems, sometimes as an early-stage scaffold and sometimes as a key intermediate along a synthetic route.
Most samples show a powdery or fine crystalline texture, and those working in a lab quickly see its stability under standard storage—room temperature and a dry, sealed bottle. It does not dissolve easily in all solvents; polar aprotic solvents like DMSO or DMF help, but even then, patience is needed. Odor remains faint, but one whiff tells any chemist that sulfur is present. Melting point tends to range from 176°C up to 182°C, hinting at purity or side products depending on synthesis. The presence of sulfur and nitrogen in two different rings means the molecule acts as both a nucleophile and an electrophile, depending on conditions. It can donate and accept hydrogen bonds, bringing it into the sights for bioscience work.
Material from reputable suppliers comes with Certificates of Analysis, typically boasting purity above 98%. Labels call out the CAS number (13965-03-2) and include clear warnings about dust inhalation, potential eye irritation, and avoidance of strong acids or oxidizers. Chemical drums or vials display pictograms meeting GHS standards—flame over circle for mild oxidative potential, exclamation mark for irritant risk. Pack sizes range from grams for laboratory research up to hundreds of grams for pilot plant testing. Each shipment leaves the vendor with a data sheet covering melting point, NMR data (proton and carbon), elemental analysis, and, for some suppliers, HPLC purity.
In the lab, most chemists rely on a condensation approach. They start with 4-aminopyridine, react it with carbon disulfide in the presence of a mild base such as potassium carbonate, and couple this to an alpha-haloketone. Temperature control makes or breaks the yield; sticking between 60 and 80°C gives the best results without too much side product. After several hours, the product precipitates and can get filtered, washed, and recrystallized. Some teams streamline the process, using microwave-assisted synthesis or different catalysts. Chemists new to this arena might need time to perfect the conditions, but once the approach is dialed in, batches show strong yields and decent purity.
This molecule stands out as a flexible building block. The thione group reacts with electrophiles to produce a range of thioethers—useful in agrochemical and pharmaceutical routes. Oxidizing agents turn the thione into a sulfoxide or sulfone, each opening new chemical directions. The pyridine ring allows for N-alkylation, while the thiazole can undergo chlorination or bromination under controlled conditions. When exposed to palladium-catalyzed cross-coupling, the compound supports Suzuki or Sonogashira-type strategies, letting researchers build more complex frameworks for medicinal chemistry. On the bioorganic side, its nitrogen and sulfur atoms make the compound a candidate for metal chelation, often in the search for new catalysts or diagnostic imaging tools.
In the chemical literature, 4-(4-Pyridyl)-3H-Thiazole-2-Thione appears under several synonyms: 2-Thioxo-4-(4-pyridyl)-3H-thiazole, 4-(4-pyridyl)thiazole-2-thiol, and 4-(4-pyridyl)-2-thioxothiazoline. Its IUPAC name and CAS registry number allow researchers to avoid confusion during ordering or citation. Chemical supply houses sometimes add their own catalog numbers, but the core identifiers remain the same. Reliable suppliers usually provide structural diagrams and translations into multiple languages, so researchers across the globe can source the same molecule for their work.
Experience in the lab has taught me that compounds with thione and pyridine groups demand respect. Lab technicians must wear gloves and goggles and keep the work under a fume hood. MSDS sheets highlight the risk of skin and respiratory irritation, especially if the compound gets airborne as a dust. Decontamination with standard soap and water suffices, and any spills get swept with damp paper to avoid kicking up dust. Waste disposal follows local hazardous regulations—not down the drain, but sealed up and sent for incineration or chemical waste processing. Fire safety measures stay close at hand, although powder fires in this context remain unlikely under good lab practice.
This thiazole thione carves out a niche in several fields. In the pharmaceutical sector, it becomes a lead scaffold for drugs targeting microbial infections, inflammation, and cancers. Some medicinal chemists use it to create kinase inhibitors or bioactive ligands. Agrochemical research taps into the same skeleton for plant protection agents—compounds that weed out pathogens or pests without harming crops. In catalysis, both academic and industrial chemists use thiazole thiones for making ligands that pull metals into productive partnerships, sometimes pushing the envelope for green chemistry goals. In analytical science, the compound pops up in sensor design, serving as a recognition element for metal ions or redox-active species.
Walking through a university research complex, one can spot this molecule used in advanced projects—graduate students test new reaction conditions, combinatorial chemists tie it into libraries for screening, and postdocs investigate how small changes alter its reactivity. Companies focus on streamlining synthesis, reducing waste, and greener routes—for example, by cutting out harsh reagents or recovering solvents for reuse. Computational chemists run modeling studies to predict electronic properties, offering guidance for where modifications might boost activity or stability. Journals publish new crystal structure data or show activity against emerging biological targets. The drive never really slows down, because each discovery with this molecule often leads to new questions and areas to explore.
Safety remains an ongoing concern. Early toxicity screens show that while the compound itself does not trigger alarm bells for acute effects, longer-term exposure data remains rare outside laboratory animals. Biological tests suggest that derivatives may inhibit enzymes involved in mammalian cell growth, which can be a boon for cancer research but raises questions for environmental safety. Studies on aquatic toxicity indicate low solubility and low bioaccumulation risk, yet environmental chemists keep tabs on any thiazole compounds getting into waterways. Most human exposure occurs in research or manufacturing settings, where protocols reduce risk to nearly zero, but as potential applications expand, toxicology will demand new investment and more transparent review.
The future for 4-(4-Pyridyl)-3H-Thiazole-2-Thione sits in the hands of those who bridge chemistry and biology—those hunting for new drugs, new materials, or better crop protection. Synthetic methods keep getting more efficient, with green chemistry helping to knock down the need for hazardous solvents. As automation comes to organic synthesis, scalability becomes less of a barrier, and this molecule can go from the milligram to kilogram scale without a sea of waste. Scientists working with artificial intelligence tools are starting to predict which derivatives will work best for certain tasks, moving quickly from theoretical designs to tangible results. Over the next decade, expect this molecule to pop up in more patents, journal articles, and maybe even in new medicines or agrochemicals that affect daily life on a global scale.
Hands-on lab experience has shown me that complex organic molecules like 4-(4-Pyridyl)-3H-Thiazole-2-Thione hold stories in each atom. At first glance, the name seems a tangle of syllables, but the structure reveals far more: a tight knit between a pyridine ring and a thiazole framework, capped with a thione group. All told, this means the molecule’s chemical formula is C8H6N2S2.
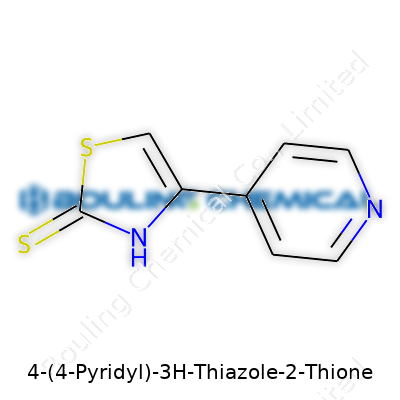
The backbone comes from the thiazole: a five-membered ring with both nitrogen and sulfur atoms. Add on a thione group at the second carbon, and the ring shifts from simple to reactive. Thione groups give the molecule both potential in synthesis and a bit more stubbornness during lab work. Attached to the fourth carbon is a pyridyl group, taken from pyridine—another six-membered ring but built with nitrogen in place of one carbon. Put it together, you get C8H6N2S2. The count stacks up: eight carbons come from the rings, six hydrogens fill the gaps, and two each for the nitrogens and sulfurs built into the thiazole, thione, and pyridine units.
Lab safety and chemical design both hinge on knowing the right molecular formula. Getting a single value wrong—one carbon out of place, a hydrogen missing—changes the entire substance. Precision chemicals like this one often become part of pharmaceutical or biotech research. Studies on similar thiazole-thione compounds have flagged them for their role as building blocks in drug discovery, anti-inflammatory research, or even imaging applications. Accuracy in identifying C8H6N2S2 means experiments can be trusted, batches don’t get mixed up, and labs keep running with fewer surprises.
From an industry perspective, small errors in formulas can lead to big headaches downstream. Quality control teams often screen raw chemicals not just for purity but for matching the declared formula. A misstep at this stage can mean time wasted, resources lost, and inaccurate or unsafe products. Several recalls traced back to ingredients that failed to match structural expectations. Clear communication around the chemical formula avoids this mess.
I remember early days in the chemistry lab, where a single typo on a formula led to extra hours ruined for me and my classmates. Sometimes, the wrong formula could even produce hazardous reactions. This isn’t unique; the literature is full of stories about near-misses due to transcription errors. Documenting things clearly and checking formulas—including C8H6N2S2—has become a non-negotiable part of the workflow for reputable research teams.
Modern cheminformatics platforms make cross-checking easier, but the human eye and a steady hand writing out each part of a molecule still matter. Coupled with strong verification practices, rigorous reference tools, and accessible databases, accuracy at the formula level reduces repeat work and keeps handlers safe. For a compound like 4-(4-Pyridyl)-3H-Thiazole-2-Thione, that starts with declaring C8H6N2S2 and double-checking every time the substance changes hands.
Clear data sheets, open-access databases, and proper chemical labeling support researchers trying to get their hands on specialty compounds. Standard bodies like IUPAC give guidance on naming and structure, but the day-to-day reality is about giving every technician, researcher, and quality control expert the right starting point—the formula. For most, the journey with this molecule begins and ends with those 8 carbons, 6 hydrogens, 2 nitrogens, and 2 sulfurs—connected not just on paper, but in practical lab and industrial experience.
Ask anyone who spends a lot of time in chemical research labs and they'll tell you that some compounds pop up across several projects because of their versatility. 4-(4-Pyridyl)-3H-Thiazole-2-Thione fits in that category. You won't find it as a household name, but researchers turn to it again and again, especially in the early stages of drug discovery, chemical synthesis, and analytical method development.
Every new medicine starts off with small steps. Medicinal chemists often hunt for molecules that offer a scaffold for building new drugs, and this compound regularly shows up on those lists. Its thiazole-thione and pyridyl components give it flexibility, making it a favorite for researchers building complex molecules to test for antibacterial or anticancer properties. Paying attention to scaffold selection early impacts the outcome down the line, as it shapes everything from biological activity to how the drug gets metabolized. Studies point to thiazole derivatives like this one playing a key role in the creation of kinase inhibitors, which sit at the core of many cancer treatments right now.
Experienced chemists like having tools that allow reactions to go places standard building blocks just can’t reach. 4-(4-Pyridyl)-3H-Thiazole-2-Thione offers just that. With its two reactive sites – a pyridyl group and a thione group – scientists use it to join together metal atoms in the formation of coordination complexes. These complexes sometimes trigger new types of chemical reactions or serve as catalysts. Over the last decade, academic research has published results where this compound worked as a ligand for copper or palladium ions, streamlining bond formation reactions. These developments matter because better catalysts often mean greener and more efficient processes.
Trying to detect metal ions or study enzyme activity? This compound doesn’t just sit quietly in the background – it plays an active role. The combination of sulfur and nitrogen atoms inside its structure makes it sensitive to metal ions in solution. Analytical chemists harness this property to develop new sensors or colorimetric assays. I’ve seen setups where a small change in color pinpoints lead, mercury, or copper in a test sample. That matters for environmental monitoring, food safety studies, and clinical diagnostics. Access to reliable chemical sensors helps labs check contamination levels or trace metals with more confidence and at a lower cost.
With all this promise comes a set of hurdles. Thiazole derivatives sometimes create stability problems, either because of their tendency to decompose or react with atmospheric moisture. Careful storage and handling are non-negotiable if you want reproducible results. Scaling up production can be tricky as well, particularly for pharmaceutical companies that need pure, safe material for further testing. Looking to the future, collaboration between synthetic chemists and process engineers could smooth out these challenges, much like cross-functional work in other scientific fields has driven new breakthroughs.
Small molecules like 4-(4-Pyridyl)-3H-Thiazole-2-Thione shape the direction of research, even if they don’t get much attention outside the scientific world. Anyone who spends their days working to create new medicines, discover cleaner chemical processes, or improve how we detect contaminants knows the practical importance of reliable chemical building blocks. For most researchers, they aren’t just tools – they’re stepping stones to solutions that matter for public health, industry, and the environment.
4-(4-Pyridyl)-3H-Thiazole-2-Thione, a mouthful for many, actually packs some valuable lessons for folks who work in labs or study chemistry. I remember my early days learning organic chemistry when my professor handed us molecules with long, complex names. Most of us groaned, but soon I realized that every ring, atom, and functional group stitched into a name wasn’t just complex jargon — it all shaped how that molecular piece behaved. For someone handling a thiazole with a pyridyl group, getting down to basics like molecular weight isn’t busywork. It holds the key for almost every step of an experiment — from measuring doses to analyzing how these molecules interact in a biological assay.
With a formula of C8H6N2S2, this molecule deserves some attention. Carbon atoms give 12.01 g/mol each, hydrogen 1.008, nitrogen 14.01, and sulfur 32.07. Add them together for eight carbons, six hydrogens, two nitrogens, and two sulfurs, and you land at approximately 194.28 g/mol. I’ve had to double-check calculations like these countless times, especially when a single decimal point changes everything. Any miscalculation means your measurements get thrown off — potentially invalidating entire batches of research. I’ve seen my classmates get tripped up by this, especially during synthesis or chromatography work where precise weights keep experiments on track.
In drug research, accurate molecular weights aren’t only for academic satisfaction. This value flows into every calculation of concentration, dosing, and reaction design. Take stoichiometry, for instance. If you start with a wrong number, you set off a domino effect in further experiments. In the pharmaceutical industry, the regulatory framework doesn’t just ask for accurate molecular weights — it expects unwavering proof that every calculation, every sample, traces back to reliable values. Auditors dig into these numbers because a misstep can compromise safety.
The molecular weight impacts solutions’ preparation, purification routines, and even equipment settings. Using 4-(4-Pyridyl)-3H-Thiazole-2-Thione as a ligand or building block for new compounds? No margin for error. Animal studies, for example, rely on accurate dosing to avoid both toxicity and wasted resources. If you don’t factor in that molecular weight, you may wind up with animals getting overdosed or underdosed, skewing results and causing ethical headaches. I’ve been in meetings where even a 1% uncertainty sparked debates among chemists and bioengineers.
Some might think it looks like a trivial detail, but getting molecular weights right cuts down on wastage. Imagine ordering 10 grams of a compound and realizing your calculations jumped by five percent. Extra expense, extra time, maybe even scrapped experiments. Cross-disciplinary research brings in large teams, and each part counts on basic information like this. In my experience, reliable, double-checked facts — especially fundamentals — build trust between chemists, engineers, and folks responsible for scale-up manufacturing.
Using digital tools for calculations can save time, but the responsibility stays on the researcher to understand the math, spot-check results, and know the implications for each project. I’ve never regretted taking a moment to double-check the breakdown of atomic weights during a busy workflow. For students and professionals alike, these habits create a foundation for everything that follows, whether synthesizing a new compound or designing a drug trial.
Every lab worker knows the tension when opening a reagent bottle only to find discolored dust or a clump where there ought to be powder. Small missteps in storage ruin entire experiments, dent tight budgets, and eat away at precious research time. Chemicals like 4-(4-Pyridyl)-3H-Thiazole-2-Thione command respect in this regard. This thiazole derivative plays a key role in chemical research, especially for those chasing discoveries in pharmaceuticals and material science. Storing it in a careless way leads to unreliable results or, even worse, safety problems few want to deal with.
Most experienced chemists treat organic thiones as vulnerable to air and moisture. Open bags of these compounds slowly lose punch over weeks, even if the losses aren’t visible to the naked eye. For 4-(4-Pyridyl)-3H-Thiazole-2-Thione, a dry, cool, and dark environment makes a solid foundation. At our university, we kept similar compounds in tightly sealed amber vials tucked away inside desiccators or refrigerators.
Light often messes with sensitive heterocyclic compounds, sometimes breaking them down or kicking off side reactions nobody wants to study. A standard shelf doesn’t protect against the effects of corrosion or the slow creep of humidity. Investing in vials with screw caps and seals gives peace of mind—no more crusty residues or mysterious color changes.
Room temperature doesn’t work for every chemical. If the storage space feels stuffy or humid, degradation ramps up. At our lab, a temperature-controlled fridge—settled around 2 to 8°C—helped extend shelf life. Some think freezing offers even more protection, but for compounds like this thiazole-thione, excessively low temperatures risk condensation and physical changes during thawing.
Air exposure is a slow poison for many thione-based molecules. We kept small repack tubs of silica gel beads in the storage box, always swapping them out if they looked pink or saturated. Even small leaks let oxidation and hydrolysis creep in, so checking seals should be a simple ritual. You can tell a lot about the compound’s shape by whether it still flows smoothly from the vial or has started caking into corners.
Ignoring storage guidelines means more than lost purity. It risks personal safety, too. Spills of degraded substances sometimes smell harsh or irritate skin, telling you something broke down inside. Storing 4-(4-Pyridyl)-3H-Thiazole-2-Thione away from water, strong oxidizers, and acids gives extra security. Accidental contamination turns a single helpful powder into a hazard or a dead end.
Researchers I’ve worked with often mark vials with the opening date, log routine checks, and track temperature trends with simple sensors. Transparency and good records let anyone spot a problem before it costs a year’s work.
Chemicals deserve as much care as the reactions they're used in. Storing 4-(4-Pyridyl)-3H-Thiazole-2-Thione well isn’t fussy—it’s practical. Keeping things dry, cool, shielded from light, and properly sealed supports reproducibility in every experiment. Good storage habits save both money and headaches, and turn careful chemists into trusted sources for quality research.
Some chemicals carry risks far beyond the beaker. 4-(4-Pyridyl)-3H-Thiazole-2-Thione is a mouthful to say, but its hazards boil down to a basic truth: treating every compound with respect keeps people safe. Despite a name that could trip up even veteran chemists, this compound doesn’t pop up in household items. Chemistry researchers, material scientists, and pharmacy developers are more likely to cross paths with it.
The scientific literature doesn’t hand out flashy warnings about massive disasters tied to 4-(4-Pyridyl)-3H-Thiazole-2-Thione. That says a lot, but it doesn’t mean it’s a free pass for careless handling. Looking at its structure, with pyridine and thiazole rings, there’s reason for caution. Compounds containing thiones and heteroaromatics can irritate the skin, eyes, or lungs. Breathing in the dust could bring coughing or headaches. Getting it on bare skin or in eyes might sting or inflame tissue. Swallowing it isn’t an experiment anyone needs.
Thiazoles can react unpredictably, especially under heat or with strong oxidizers. The thione group itself raises questions about long-term toxicity. Getting exposed to these chemical types, even at low levels, sometimes stacks up subtle health effects. That’s where experience matters—it taught me early on to respect even the least glamorous bottles with proper gloves and a keen eye for spills.
I remember the day a seasoned colleague—an old-school bench chemist—brushed off standard gloves during a routine synthesis. “This stuff isn’t cyanide,” he joked… until a splash caught his wrist and left a red mark that lingered for weeks. Small mistakes in chemical handling build bigger problems, both for personal health and for the work environment. Tiny exposures might start with irritation but can turn chronic if repeated over time.
The US Occupational Safety and Health Administration (OSHA) doesn’t label this compound as a major threat. Still, university labs and pharma companies treat it like a chemical with unknown dangers, using fume hoods and personal protective equipment (PPE). 4-(4-Pyridyl)-3H-Thiazole-2-Thione isn’t listed on every hazard database, but that only reinforces the need for caution: the unknown is sometimes more dangerous than the notorious.
Working with chemicals, I learned early that no shortcut beats consistent safety. Never work with powders or unfamiliar reagents outside a fume hood. Keep nitrile gloves on, eye protection well-fitted, and lab coats buttoned. Spills get cleaned right away, without excuses. Once a bottle gets opened, a tight lid goes back on—nothing left to chance. If a material safety data sheet (MSDS) doesn’t hand over all answers, ask the company’s health and safety officer or look to peer-reviewed studies for new data.
Stocking labs with proper safety gear, keeping emergency eyewash stations ready, and making training routine work better than warning posters. Anyone handling chemicals like 4-(4-Pyridyl)-3H-Thiazole-2-Thione should see enforced safety not as a task, but as a culture. Every person who sets foot in a chemistry lab owes it to themselves and their team to treat every bottle as if it could end a career—or worse.
Better habits protect more than individuals—they support accurate research and reliable products. Regular lab audits, up-to-date training sessions, and strong reporting channels for near-misses help prevent small mistakes from turning into emergencies. Real protection doesn’t rest on luck or lenient rules; it springs from everyone buying into safety. Chemicals with tricky names or unclear risks belong in the same cautious category as the obvious dangers—no exceptions.
Safety practices learned with 4-(4-Pyridyl)-3H-Thiazole-2-Thione broaden out into future work. Habits, not luck, keep science moving forward safely. If you work in the field, the wise step is never rolling the dice when a simple pair of gloves, eye protection, and a clean, organized bench can dodge a world of hurt.
| Names | |
| Preferred IUPAC name | 4-(pyridin-4-yl)-3H-1,3-thiazole-2-thione |
| Other names |
4-(4-Pyridyl)-2-thioxo-1,3-thiazolin-3-one NSC 412465 |
| Pronunciation | /fɔːr fɔːr pɪˈrɪdɪl θaɪˈæzəʊl tuː ˈθaɪəʊn/ |
| Identifiers | |
| CAS Number | 72058-18-3 |
| 3D model (JSmol) | `3DModel: JSmol "C1=CN=CC=C1C2=CSC(=S)N2"` |
| Beilstein Reference | 126319 |
| ChEBI | CHEBI:147040 |
| ChEMBL | CHEMBL152962 |
| ChemSpider | 21877239 |
| DrugBank | DB08320 |
| ECHA InfoCard | 06d702a3-fd32-40b3-b1eb-2c6b84566289 |
| Gmelin Reference | 135226 |
| KEGG | C11131 |
| MeSH | D000068612 |
| PubChem CID | 657308 |
| RTECS number | RN8825000 |
| UNII | JI898MEG69 |
| UN number | Not regulated |
| CompTox Dashboard (EPA) | DTXSID2061278 |
| Properties | |
| Chemical formula | C8H6N2S2 |
| Molar mass | C8H6N2S2 = 194.28 g/mol |
| Appearance | yellow powder |
| Odor | Odorless |
| Density | 1.47 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.14 |
| Acidity (pKa) | 5.2 |
| Basicity (pKb) | 7.93 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.7100 (estimate) |
| Dipole moment | 3.85 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 230.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed or inhaled. Causes skin and eye irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P261-P280-P305+P351+P338-P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0-0 |
| LD50 (median dose) | LD50 (median dose): >5000 mg/kg (oral, rat) |
| REL (Recommended) | 22-25°C |
| Related compounds | |
| Related compounds |
4-(4-Pyridyl)thiazole 4-(4-Pyridyl)-2-aminothiazole 4-(4-Pyridyl)-2-thioxothiazolidin-3-one 2-mercaptothiazole 4-phenylthiazole-2-thiol |