Chemistry always advances through curiosity and necessity. 4,4-Piperidinediol, Hydrochloride started popping up in laboratory journals and industrial notes around the mid-20th century. The molecule’s simple structure didn’t keep it out of the limelight—the diol attached to a piperidine backbone gave it a good hook for further tweaking. Labs noticed its potential both as a direct agent and a chemistry building block long before the compound really reached widespread catalog recognition. By the 1970s, improved synthetic procedures and a better understanding of its reactivity opened up new possibilities for chemical and pharmaceutical work. Now, papers and patents mention it both for large-scale industry and for fine-tuned research, especially as work in heterocyclic chemistry continues to grow.
4,4-Piperidinediol, Hydrochloride gets manufactured in a crystalline, white or off-white form. Its main draw lies in the two hydroxyl groups at the 4-position of the piperidine ring, sitting neatly for reaction or modification. Producers package it for shipment under dry and airtight conditions due to its affinity for water. Materials safety data sheets and technical bulletins notify chemists and technicians about storage—dry, cool areas in sealed containers prevent clumping and degradation. It’s often sold in bottle sizes sized for both research and large processing; suppliers target pharmaceutical labs, academic researchers, and specialty chemical production outfits.
This hydrochloride salt forms sturdy, fine crystals. Its melting point ranges near 205–210°C, depending on sample preparation and moisture content. Water brings it into solution; organic solvents do so less efficiently due to the salt form. As a hydrochloride, it boasts higher stability under ambient conditions compared to its neutral form. Acidic pH tie-in means chemical transformations use buffered environments. The molecule is stable under recommended storage, but does not resist strong oxidizers or bases over extended periods. Odor-free and bland to the touch, it slips through analytical processes—HPLC, NMR, FTIR—without generating noise or excess peak clutter, making purity checks feasible for labs of all sizes.
Labs checking certificates of analysis for 4,4-Piperidinediol, Hydrochloride look for the minimum purity—above 98% serves high-end synthesis or regulatory work. Sodium, potassium, chloride, and other contaminant levels come in under parts-per-million thresholds, preventing interference in further synthesis. Labels follow global chemical standards, giving the UN number and hazard class, verified CAS registry number, and all recommended handling symbols. Large manufacturers also track batch traceability using barcode systems, so any recall or anomaly investigation starts and ends without guesswork. Shipment labels specify GHS pictograms so handlers spot corrosive or irritant risks at a glance, even if language barriers exist.
Labs obtain 4,4-Piperidinediol, Hydrochloride through reduction and protection cycle methods. A common approach starts from glutaric acid derivatives, where cyclization delivers the six-membered ring. The 4,4-dihydroxy orientation comes from oxidative or selective reduction, often using sodium borohydride under carefully monitored pH. To secure the hydrochloride, freebase product gets treated with hydrochloric acid in water, then isolated by slow evaporation or precipitation with an anti-solvent like acetone. The resulting salt is filtered, washed, and dried under vacuum—a crucial step to finish with a product meeting tight specification. Labs scale this workflow from a few grams to tens of kilograms, so anyone from an undergraduate chemist to a chemical engineer can use or adapt it.
Having two hydroxyl groups opens up 4,4-Piperidinediol, Hydrochloride to esterification, etherification, and oxidation. The piperidine ring resists acid and base hydrolysis, yet delivers selectivity to ring-substitution, making it attractive for custom synthesis. Reagents like acyl chlorides readily react with the diol for ester analogs, while alkylation under controlled conditions leads to ether formation if needed downstream. The hydrochloride's protonation state lets reactions proceed in staged, predictable pathways, keeping byproducts to a minimum. Many pharmaceutical projects use it as an intermediate in the assembly of CNS drugs, either by adding extra groups or as a vector for more complex heterocyclic scaffolds.
4,4-Piperidinediol, Hydrochloride often appears in literature or catalogs under synonyms like Piperidine-4,4-diol hydrochloride, 4-Hydroxypiperidine hydrochloride, or even by registry numbers such as CAS 40064-34-4. Certain patent filings and older synthesis references use less common tags or notation systems, so researchers checking compatibility or availability know to cross-reference. Manufacturers sometimes introduce internal codes or abbreviated labels for inventory; for regulatory filings, full nomenclature—including all systematic identifiers—remains standard practice. A search through chemical supply or regulatory databases confirms multiple synonym paths end at the same physical compound, preventing confusion or duplication of inventory.
Lab work needs practical safety rules. 4,4-Piperidinediol, Hydrochloride, like many amine hydrochlorides, asks for gloves, eye protection, and ventilation. Material safety data point to low volatility: no routine respiratory risk unless dust becomes airborne. Skin and eye irritation land among potential concerns if direct exposure occurs, pushing labs to rely on goggles and fume hoods. If anyone spills it, standard chemical absorbents and triple-rinse cleanup protocols apply. Waste disposal channels this material to solvent or halide waste streams; municipal sewers or general trash aren’t options. First aid sections in safety documentation explain prompt flushing with clean water and medical attention for accidental contact, showing a clear trail from accident response to professional care.
Research and production teams turn to 4,4-Piperidinediol, Hydrochloride for its unique nitrogen and oxygen chemistry. Drug discovery projects use it to probe CNS-modifying structures or for opioid receptor ligand platforms. Organic synthesis labs favor its reactivity in heterocyclic assembly lines, particularly if they need a non-planar, hydroxyl-rich intermediate, ideal for stepwise chemical design. It also sees use as a building block for custom monomers in polymer and material science. Some technical papers chart out radical scavenging and antioxidant studies, suggesting utility in specialized biochemical assays. The compound doesn’t appear on major regulated drug precursor lists, making it more approachable for above-board research channels.
Big-picture and small-scale R&D both rely on standard intermediates for rapid prototyping. 4,4-Piperidinediol, Hydrochloride fits into drug substance development, sometimes as a direct precursor, sometimes as a side-ring for testing SAR patterns. Analytical chemists map its reaction behavior with contemporary instrumentation, giving rise to published NMR, MS, and X-ray crystallography data. Advances in solid-phase and flow synthesis have re-evaluated its utility by cutting waste and reaction times. The molecule even features in preclinical toxicology sets, helping screen for off-target effects long before scale-up. New derivatives and analogs built on this frame power patents and push frontiers in both academic and industrial chemistry.
Toxicologists require data on new and established chemicals. Animal and cell-culture models show that 4,4-Piperidinediol, Hydrochloride doesn’t behave like more dangerous or reactive piperidine analogs, but does pose moderate risk of irritation or acute toxicity at high or prolonged exposures. Oral LD50 values point to low-millimole scale risk zones, with main symptoms confined to the gastrointestinal track or mild CNS depression at very high doses. Regulatory dossiers, including REACH documentation and global hazard labeling, call out eye and skin irritation, making standard precautions necessary for all users. Chronic exposure data remain rare; as a result, occupational safety authorities suggest rotation and exposure limits while keeping emergency eyewash stations and clean facilities on hand. No major carcinogenic or mutagenic signals appear in current literature, yet prudent practice dominates professional recommendations.
As chemistry moves toward personalized medicine and efficient industrial routes, 4,4-Piperidinediol, Hydrochloride stands ready to fill roles as a starting point for targeted synthesis. Green chemistry frameworks look to optimize its preparation with leaner, less polluting processes. The diversity of potential modifications and the expanding set of analytical tools point toward more specialized derivatives in neurological drugs and smart polymers. Earlier restrictions on precursor molecules for controlled substances drive continual review and clarification in regulatory circles, but transparency and proactive safety research position it for long-term use in above-board science. As more labs trade experience through open publications and better electronic data systems, the knowledge ecosystem surrounding this compound will only grow, helping next-gen chemists and engineers solve problems with reliability and precision.
People outside the chemistry world probably won’t spot 4,4-Piperidinediol, hydrochloride at a pharmacy or supermarket. Chemists know it well for its role as an intermediate. Over the years, it’s become a building block in labs and manufacturers’ toolkits, especially in the synthesis of medicines and certain specialty chemicals.
Drug makers sometimes use it during the process of creating painkillers, muscle relaxants, or drugs for the nervous system. Its unique structure plays a supporting role in carving out the backbone of molecules with activity in the human body. Most folks won’t see this compound on its own but might have benefited from products that started with it at the raw material stage.
Law enforcement agencies keep a close eye on 4,4-Piperidinediol, hydrochloride because it can serve as a precursor for illicit substances, especially synthetic opioids. In recent years, the opioid crisis has highlighted how substances found in normal labs can become sources for dangerous drugs. The US Drug Enforcement Administration, for instance, lists it among chemicals flagged for potential misuse.
Many regulatory agencies set rules around buying, handling, and tracking it. Labs and businesses purchasing this compound follow strict protocols, including registration and reporting. Anyone working with it needs to document every step, showing both where it goes and where it came from. This approach helps cut off illegal channels before problematic compounds reach the streets.
My own time working with chemical suppliers showed me how easily these substances pass from hand to hand in the supply chain. I remember conversations with chemists who don’t just check off safety boxes but review every source and shipment, aware of the broader picture. For every reagent, especially those with dual uses, there’s a story: one meant for discovery and therapy, another shadowed by the threat of diversion. Few situations drive home the responsibility like handling a substance flagged by regulators.
Education in proper handling, storage, and disposal isn’t just a legal hoop—lives depend on those practices being airtight. The temptation for some to save time or money by loosening records or skipping training has led to real harm. A single slip-up in the chain of custody can end up making headlines for all the wrong reasons.
Turning the tide means more than tracking chemicals. Supporting better communication between manufacturers, researchers, authorities, and health experts could close gaps that criminals exploit. Modern database systems that quickly flag unusual purchases or inconsistencies could tip off regulators before problems escalate. Training shouldn’t feel like a chore but include real examples from recent events, bridging the classroom with reality.
Encouraging dialogue between the chemistry world and the wider public helps dispel myths and build trust. People deserve to know how labs operate and work to protect communities, especially where chemical supplies overlap with public health. At every step, honest discussion about both promise and peril shapes better policies and safer outcomes.
Talk of chemicals doesn’t always scare people away. Still, once a substance starts showing up in law enforcement’s reports or regulatory lists, it’s smart to wonder what makes it so special—or dangerous. A chemical called 4,4-Piperidinediol, hydrochloride (sometimes shortened to PHD) pops up in both scientific literature and government documents for a good reason. Its story connects not just chemistry, but questions about safety, crime, and public health.
Some chemicals fly under the radar for decades. Not this one. Agencies like the U.S. Drug Enforcement Administration (DEA), the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), and Health Canada monitor 4,4-Piperidinediol, hydrochloride. Why? It’s classified as a List I chemical in the United States, meaning it’s tightly controlled. Put plainly, this compound acts as a key piece in the illegal manufacture of certain opioids, including fentanyl and its derivatives. Fentanyl-related overdoses take thousands of lives every year, so tracking and controlling building blocks like PHD matters.
My years working with public health advocacy introduced me to the ways policy can lag behind reality. The opioid crisis hit hard, partly because labs found ways to skirt the rules. Substances like 4,4-Piperidinediol, hydrochloride landed on enforcement radars only after real damage appeared in hospitals and communities. Handling it requires protective gear—at minimum gloves, goggles, good ventilation—because the pure substance can irritate skin, eyes, and the respiratory tract, or worse if it gets ingested or inhaled. The real trouble, though, comes from its criminal use.
Governments respond with new rules once a threat becomes clear. In the U.S., registering with the DEA is the only way to legally possess or distribute 4,4-Piperidinediol, hydrochloride, and each step—import, export, use—gets logged. Europe and Canada have established similar access controls. Legitimate scientific or medical work around this chemical must go through tons of paperwork, audits, and checks. All that bureaucracy can slow down research, but the alternative gives organized crime a wide open field. It's a balancing act between scientific freedom and limiting the spread of fentanyl and similar synthetic opioids.
Real solutions rarely come from a ban alone. I’ve seen local communities pressed to do more, but without the proper resources, it’s a losing battle. Stopping the flow of PHD won’t solve opioid addiction; demand creates supply. Still, choking off access to precursor chemicals forces illegal labs to work harder, making it riskier and less profitable. Tighter border inspections, international agreements, and regular updates to restricted chemical lists help slow the spread, but this effort costs money and takes cooperation across borders and professions.
Industry needs to play its part, too. Chemical manufacturers and distributors have a part in this story. Real, up-to-date tracking systems and strong customer vetting cut down the odds of chemicals falling into criminal hands. Public education helps as well—showing people where real threats begin, not just where they end. Shaping effective responses means public health agencies, scientists, law enforcement, and business each pulling their own weight.
4,4-Piperidinediol, hydrochloride isn’t a household name, yet the role it plays behind the scenes puts lives at risk. Ignoring the details invites new problems; getting obsessed with red tape slows research that could help. The challenge calls for steady, honest work on all fronts—not just another item on a list, but part of a broader fight for safety, compassion, and clear-eyed science.
4,4-Piperidinediol, Hydrochloride isn’t something most folks run into unless they work in a laboratory or manage a chemical storeroom. Still, it’s more than just another compound on the shelf. I’ve seen a lot of talk about synthetic chemicals and the headaches they can cause if not kept in the right conditions, and this one’s no exception. This isn’t table salt; a little carelessness can trigger a chain of problems nobody wants to handle.
This chemical is listed for restricted use in several countries, and for good reason. Authorities highlight its potential to serve as a precursor in illegal drug manufacturing. My years in the lab taught me that slipping on storage isn’t an honest mistake—it’s an open invite for legal headaches and safety scares. I remember a colleague’s story: a small leak from a poorly sealed container turned into an all-night cleanup. The stress in that room was thick enough to taste.
4,4-Piperidinediol, Hydrochloride doesn’t mix well with heat, moisture, or open air. I always insist on keeping compounds like this in air-tight, non-reactive containers—glass or certain plastics work well. Screw caps should seal tight. Every time I worked late in the chemical storage room, I checked containers for even the faintest sign of corrosion or residue. Even a tiny compromise matters over time.
Dry, cool storage makes a huge difference. Warm spots speed up decomposition or even react with other substances, occasionally throwing out some kind of gas or heat that you do not want. I’ve dealt with containers sweating in humid rooms, and one thing is clear: always run a dehumidifier if the air feels sticky. Always avoid windows or spots exposed to sunlight, since UV can kickstart unwanted reactions.
I never trusted unlabeled bottles, not after seeing one mix-up that almost ruined some research and sent a grad student to the campus clinic. Always mark the date of receipt and who opened the container last; this isn’t overkill, it’s responsible stewardship. Regular audits cut down on surprises. I’ve found more forgotten or misplaced reagents during a Thursday inspection than I'd like to admit.
Laws around this chemical aren’t just bureaucracy—they reflect real risks. Letting unauthorized access slide creates more than regulatory trouble. There’s also an ethical side here. Staying vigilant helps keep these chemicals out of the wrong hands. I keep records locked away, and several labs require visitors to sign in before they’re even allowed in the storage room.
On the environmental side, never dump old or unneeded material. Proper hazardous waste disposal is a must. I had to sit through enough safety training to remember that improper disposal quietly poisons groundwater and creates a lasting problem for everyone.
Many labs now use digital inventory software to flag expiring chemicals and track who handled what. Training sessions sharpen everyone’s storage habits and keep fresh eyes on the details that matter. I push for open talks about near-misses—sharing stories beats reading rules on a wall any day. People learn by example, and one honest conversation about a mistake often rattles more sense into folks than another line in a manual.
4,4-Piperidinediol, Hydrochloride deserves respect all the way from delivery to disposal. Taking shortcuts here doesn’t just risk lab safety— it ripples out into the wider world. Vigilance in storage habits pays off every time in safety, compliance, and peace of mind.
4,4-Piperidinediol, Hydrochloride draws attention for anyone involved in pharmaceutical chemistry or laboratory research. This compound stands out for its unique structure and how it fits into synthetic and analytical processes. My own work with similar nitrogen-centered compounds has shown how these molecules help build more complex agents, giving practitioners in the field a clear pathway from raw material to functional product.
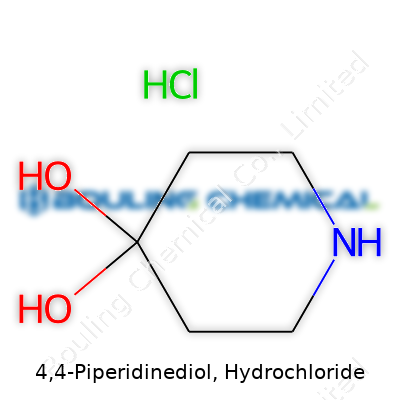
The foundation for 4,4-Piperidinediol, Hydrochloride rests in its piperidine ring. Picture a six-membered ring containing one nitrogen atom—this is not just standard organic chemistry scaffolding. The real punch comes from the two hydroxy groups at the 4-position. This makes the molecule 4,4-dihydroxy-piperidine before it even meets its hydrochloride partner in the next stage. Once hydrochloric acid steps into the mix, a salt forms, which is common practice to boost stability and solubility when developing chemicals for both research and therapeutic use.
The chemical formula of 4,4-Piperidinediol, Hydrochloride is C5H11NO2 · HCl. The parent molecule, 4,4-dihydroxypiperidine, provides the skeleton: a six-atom ring with nitrogen replacing one of the carbons, and both hydroxy groups attached to carbon number four. Adding hydrochloride changes the formula, yet the backbone stays the same—just now, the molecule holds the HCl tightly through ionic interactions. In a two-dimensional drawing, you see the piperidine ring with hydroxy groups jutting off the same carbon, while the hydrochloride portion attaches to the nitrogen, lending the compound higher solubility in water.
Having spent time at the lab bench handling heterocyclics like this one, I see firsthand how its structure influences behavior and downstream possibilities. Those hydroxy groups empower researchers to use it as a stepping-stone to create more complicated scaffolds. Everything from pain relievers to neurological agents has links to piperidine derivatives. People in research settings prefer the hydrochloride salt since it stores well and works across a range of reactions without breaking down easily. That reliability matters. Nobody wants a precursor that fights back or decomposes before the next reaction step begins.
4,4-Piperidinediol, Hydrochloride offers a solid foundation, but it doesn’t come without challenges. Depending on the supplier, purity can swing a bit, and some batches still carry unwanted residual solvents. I’ve found thin-layer chromatography and NMR spectroscopy helpful for checking quality before using it in crucial syntheses. Regulatory oversight keeps rising, so tracking provenance and handling protocols cannot be ignored. Maintaining clear documentation protects researchers and meets current expectations for chemical stewardship.
Quality matters more than ever. Good communication with suppliers pays off. I look for transparent documentation—batch history, test reports, traceability. Lab teams can create protocols for safe storage, using desiccators and lightproof containers to shield against degradation. Routine staff training on chemical handling goes beyond compliance; it prevents unnecessary risk. Investing time up front brings fewer headaches later, especially with compounds riding the edge of regulatory interest.
Whether in a university, startup, or mature chemical company, clear understanding of 4,4-Piperidinediol, Hydrochloride’s chemical makeup pays off. Knowing your compound inside out builds a foundation of trust between science and practice. This approach—combining structural knowledge with hands-on experience—keeps research moving in the right direction.
4,4-Piperidinediol, Hydrochloride shows up in some lab and industry spaces thanks to its chemical properties. Handling it without respect for its risks can turn a regular day into a bad one fast. This compound can irritate the skin, eyes, and lungs, and exposure over time grows the list of possible health problems. Scientists and workers use gloves, safety goggles, and lab coats for a reason — no one volunteers to test what happens if careless hands or faces meet this stuff. Damaged packaging or poor storage make things even worse. If your workspace gets warm or humid, that can create slip-ups you won’t soon forget.
Anybody who works with 4,4-Piperidinediol, Hydrochloride counts on reliable routines. A decent ventilation system goes a long way in stopping fumes before people breathe them in. More than one lab worker picked up a cough or burning eyes after trusting a plain room instead of using a fume hood. That stuff stays in the air longer than you think.
I’ve watched coworkers rush or skip steps to save a minute. It only takes one time handling solids or powders without proper gloves, and you pay for it. Nitrile gloves, layered with a sturdy lab coat, work as a second skin. Safety goggles don’t make anybody look cool, but protecting your eyes always ranks higher than looks. Folks who get chemical dust or splashes in the eyes risk vision damage, not just discomfort. There’s no excuse for working without easy-to-find eyewash stations or safety showers nearby.
Spills happen most often when people act casual with containers. Screw caps tight, label jars clearly, and use secondary containment like trays. Some people skip the second container, but it only takes one mistake to regret it. Accidents spill over into arguments about who’s responsible and what’s contaminated.
Disposing of anything containing 4,4-Piperidinediol, Hydrochloride asks for discipline. Never pour waste down the drain – local and national rules usually have strict requirements for this sort of chemical. Secure waste in approved, clearly marked containers. When waste adds up, certified disposal teams know what to do next. Cutting corners with disposal brings legal trouble quickly, and the risks to water sources or soil stick around long after labs close.
No equipment offers the same peace of mind as knowing what to do in an emergency. Regular drills and solid training mean fewer panicked reactions. While some people roll their eyes at monthly reminders, the first real spill shows who cared to listen. Written procedures belong up-to-date and visible in every space, with phone numbers for medical help within reach.
People need to work together, not just trust themselves. If a coworker skips steps, say something early, before their mistake becomes your problem. Supervisors need to reward careful habits, not just fast work. At the end of the day, everybody wants to head home healthy. Safety depends on shared attention, clear methods, and a willingness to follow boring but essential steps. 4,4-Piperidinediol, Hydrochloride won’t forgive sloppiness, but with the right habits and tools, it won’t catch anyone off guard either.
| Names | |
| Preferred IUPAC name | 4-hydroxypiperidin-4-ol;hydrochloride |
| Other names |
4,4-Dihydroxypiperidine hydrochloride Piperidine-4,4-diol hydrochloride 4,4-Piperidinediol hydrochloride |
| Pronunciation | /ˌpaɪˈpɛrɪdiːnˌdaɪˌɒl haɪˈdrɒklaɪd/ |
| Identifiers | |
| CAS Number | 40064-34-4 |
| 3D model (JSmol) | `3D model (JSmol)` string for **4,4-Piperidinediol, Hydrochloride**: ``` NC1CC(O)CC(O)C1.Cl ``` This is the **SMILES** string representation usable in JSmol or other cheminformatics tools. |
| Beilstein Reference | 147937 |
| ChEBI | CHEBI:132741 |
| ChEMBL | CHEMBL4186777 |
| ChemSpider | 26189069 |
| DrugBank | DB08209 |
| ECHA InfoCard | 07d1c9c1-fb60-47ac-af0c-cb1174f0261b |
| EC Number | 210-483-1 |
| Gmelin Reference | 126156 |
| KEGG | C14422 |
| MeSH | D018239 |
| PubChem CID | 16213794 |
| RTECS number | WI0140000 |
| UNII | M7D447J2ZX |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C5H12ClNO2 |
| Molar mass | 142.62 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.1 g/cm³ |
| Solubility in water | soluble in water |
| log P | -2.2 |
| Acidity (pKa) | 8.1 |
| Basicity (pKb) | 2.84 |
| Magnetic susceptibility (χ) | -61.6·10^-6 cm³/mol |
| Viscosity | Viscous liquid |
| Dipole moment | 3.02 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 128.7 J·mol⁻¹·K⁻¹ |
| Hazards | |
| Main hazards | Causes skin irritation, causes serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | Precautionary statements: P264, P270, P280, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 81.6°C |
| LD50 (median dose) | LD50 Oral Rat 984 mg/kg |
| NIOSH | WX8400000 |
| PEL (Permissible) | PEL (Permissible exposure limit) for 4,4-Piperidinediol, Hydrochloride is not specifically established by OSHA. |
| REL (Recommended) | 0.21 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Piperidine Piperidine hydrochloride 4-Hydroxypiperidine 4-Piperidone 1,4-Piperidinediol |