Chemists have spent decades searching for piperidine derivatives that open new doors in medicinal chemistry and chemical biology, and 4-(4-Iodo-1H-Pyrazol-1-Yl)Piperidine has become one of those key molecular tools. Research papers from the early 2000s show pyrazole rings making their way into drug scaffolds, often to increase binding potential or tune pharmacological properties. The addition of an iodine atom to the pyrazole ring – an approach that gained traction with advances in halogenation methods – lent this molecule a unique role in radiochemistry and diagnostics. Organic and medicinal chemistry circles took notice, particularly as targeted modification of heterocycles like this one became more mainstream.
4-(4-Iodo-1H-Pyrazol-1-Yl)Piperidine appears as a bench-stable solid, light to medium yellow in hue, but its most significant feature is the iodine moiety at the pyrazole position. This feature makes the compound valuable for further derivatization or radiolabeling. Research teams often look for molecules like this as intermediates, especially when planning synthetic schemes around kinase inhibitors, ion channel modulators, or molecular probes for imaging. The molecule stands out not just for its reactivity but also its compatibility with commonly used solvents and its integration into broader synthetic routes.
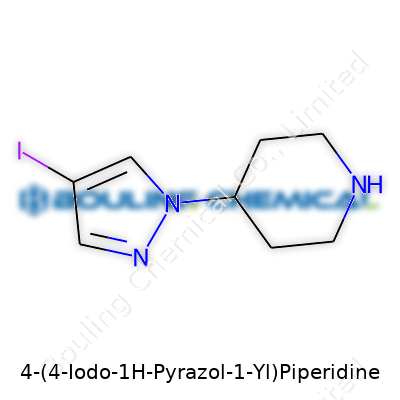
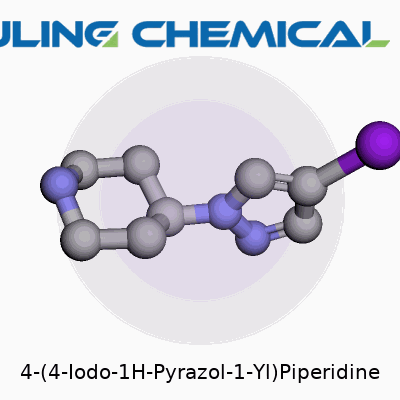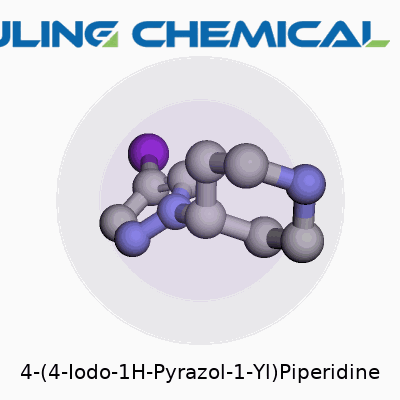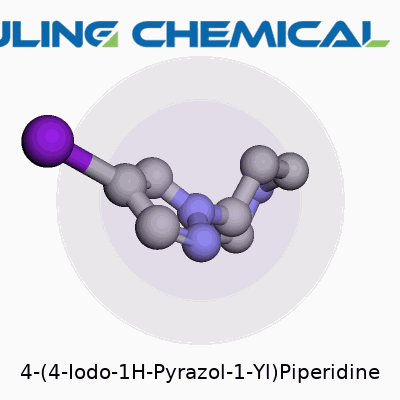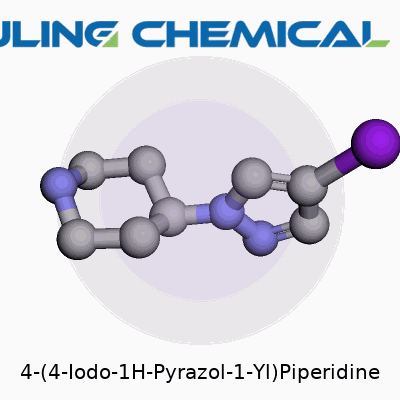
Examining the structure, we find a six-membered saturated piperidine ring linked through its 4-position to a 1H-pyrazole that holds iodine at the 4-position. Its molecular formula clocks in at C8H12IN3, and the compound’s mass, topped by the hefty iodine atom, places it at roughly 289 g/mol. Most suppliers indicate it has a melting point in the region of 112–116°C. The compound dissolves well in DMSO and DMF, less so in less polar solvents, and its logP value sits on the higher side for a heteroaromatic. Under standard lab conditions, the compound resists rapid degradation, but exposure to strong bases or oxidants can cause it to decompose or lose iodide.
Reputable sources provide 4-(4-Iodo-1H-Pyrazol-1-Yl)Piperidine as a highly pure solid, with HPLC yields above 98%. Standard labeling covers the batch number, CAS registry number, chemical structure, recommended storage conditions, and the date of synthesis or repackaging. Keeping the compound dry and protected from light at 2–8°C or ambient temperatures ensures extended shelf life. Many users appreciate clear labeling indicating potential hazard classes and GHS pictogram requirements, considering the presence of the iodine atom, which can present specific health and environmental risks.
Synthetic routes usually start from commercially available 4-iodopyrazole or a suitably protected variant. Researchers combine this building block with piperidine through N-arylation, capitalizing on palladium-catalyzed cross-coupling such as Buchwald-Hartwig amination, using a suitable base and ligand. To maximize yield, reaction conditions are tuned for temperature and solvent polarity, often favoring a mild base like potassium carbonate and a polar aprotic solvent such as DMF. Workups typically require aqueous quenching and extraction, with careful chromatographic purification to separate side products and unreacted material. Lab teams with access to automated flash systems can streamline this process.
This compound offers multiple paths forward for further chemistry. The reactive iodine atom opens the door to Suzuki-Miyaura couplings for the introduction of aryl groups, Sonogashira reactions to add alkynes, or simply radioiodination for tracer studies. The pyrazole ring can also participate in electrophilic aromatic substitution, though its activity is tempered by the electron-withdrawing iodine. On the piperidine side, the nitrogen can be alkylated or amidated, enabling the attachment of fluorescent tags, biotin, or other probes for target engagement studies. Modern labs with access to parallel synthesis platforms often use this molecule as a scaffold to build compound libraries for high-throughput screening.
Literature and suppliers might refer to this molecule as 4-(4-Iodo-1H-pyrazol-1-yl)piperidine, 1-(4-Piperidinyl)-4-iodopyrazole, or by catalog numbers from supply houses. Its systematic IUPAC name keeps popping up in journals, but for everyday lab work, chemists often shorthand it to “Iodo-pyrazole piperidine” or “I-PYP.” Regardless of the label, the structure speaks louder than the name, as any chemist familiar with these rings can spot the key reactive handles on sight.
4-(4-Iodo-1H-Pyrazol-1-Yl)Piperidine calls for careful handling, using fully enclosed personal protective equipment—lab coats, nitrile gloves, and safety goggles—thanks to its potential to cause skin and respiratory irritation. Accidental inhalation of dust, although unlikely due to its crystalline nature, should be avoided by using a fume hood. Cleaning up any spills quickly and thoroughly, using inert absorbent materials, helps keep workspaces safe. Disposal requires precise tracking, especially with iodinated waste materials, to comply with institutional protocols and local environmental rules. Researchers working with radioiodinated analogs need to follow additional isolation and monitoring measures, and emergency eye-wash stations and safety showers help address exposure.
Medicinal chemistry regularly taps into the reactivity of this molecule to build kinase inhibitors, particularly those targeting signaling proteins implicated in cancer and autoimmune disorders. Imaging applications benefit from iodine-125 or iodine-131 radiolabeling, allowing precise mapping of molecular targets in living systems, a critical piece for the diagnosis and staging of certain tumors. Chemical biologists use it as a probe handle to pull down protein complexes or for tethering to solid supports during affinity purification. In the hands of synthetic chemists, it acts as a springboard for making more complex structures with bioactive potential.
Ongoing research circles around optimizing the synthesis of this molecule to avoid harsh conditions and minimize hazardous waste. One group reported a nickel-catalyzed cross-coupling method that reduces the need for noble metals, trimming both cost and environmental impact. Scientists are trying to expand the palette of possible substitutions on both the pyrazole and piperidine rings, hoping to discover new therapeutic properties. In academic settings, this molecule keeps showing up in grant proposals and high-impact publications, especially in studies aiming to understand the relationship between molecular structure and biological activity. In drug discovery, combinatorial chemistry approaches feature this molecule as a scaffold for rapid hit-and-lead identification.
As with many halogenated heterocycles, questions about long-term safety and environmental persistence keep coming up. Toxicology panels in rodents suggest the compound shows low acute oral toxicity, but there are open questions about chronic exposure. Some in vitro assays display moderate cytotoxicity at higher concentrations, an effect most pronounced in hepatocyte lines. Research keeps exploring metabolic pathways and degradation products because iodine release can impact thyroid function. Proper labeling with hazard pictograms and safety data keeps users informed, but the push for safer analogs continues. Regulatory guidelines stress the need for detailed reporting of all incidents and side effects during laboratory use.
Looking ahead, 4-(4-Iodo-1H-Pyrazol-1-Yl)Piperidine seems set for wider adoption across both industry and academia. Expansion in the radio-pharmaceutical market will likely hinge on methods for rapid, high-yield labeling. As more teams embrace AI-driven drug discovery, the compound’s structural features fit well with in silico screening pipelines, helping to identify new therapeutic targets. Environmental chemists keep a close eye on iodine-based compounds, so future iterations may feature “greener” synthetic processes and tunable degradation profiles. Spotlighting the molecule in open-access databases should help connect chemists, biologists, and material scientists, making this compound a mainstay in modern chemical research.
In chemistry, structure tells you more than just “what it looks like.” It explains how a compound behaves, how it interacts with living systems, and where it fits in the bigger picture of science and health. Looking at 4-(4-Iodo-1H-Pyrazol-1-Yl)Piperidine, the atoms and bonds reveal the core of its identity. Take the core: a piperidine ring—six membered, saturated, with one nitrogen. Piperidine rings show up all over the pharmaceutical map. Bring in a pyrazole ring—a five-membered partner with two nitrogens, often seen in drug design for its ability to mimic natural molecules. Now, the “4-iodo” twist: an iodine atom locked onto the fourth position of the pyrazole, adding both heft and a big chemical personality.
The chemspeak version: the piperidine and pyrazole connect through their nitrogens. This puts the iodine right out on the edge, making it more than just a heavy atom tag—it’s a game changer. Iodine bites with size and polarizability. It changes how molecules interact: with proteins, membranes, and even light. Meanwhile, the nitrogen-rich system brings basicity and the chance to form hydrogen bonds. So, this isn’t just a neat string of atoms; it’s a tool for tweaking biological connections and physical properties.
Every part of this structure does some heavy lifting in medicinal chemistry. Piperidine shows up in antihistamines and antipsychotics. It’s like a comfort food for chemists, providing a backbone that the body recognizes. Pyrazole is ready to slip into enzyme pockets, block unwanted cell reactions, or act as a signal. Add an iodine, and suddenly there’s a way to use radioactivity in imaging, or to adjust how the compound dissolves and distributes in the body.
My own time in synthetic chemistry labs taught me that tiny tweaks drive giant changes. Swap out a hydrogen for iodine and watch the compound flip the script on how it interacts with proteins. Medicinal chemists spend weeks just shifting halogens and examining subtle boosts in drug strength or selectivity. Those changes mean the difference between a dud and a blockbuster treatment.
Without the right structure, molecules can’t do the job we want. For something like 4-(4-Iodo-1H-Pyrazol-1-Yl)Piperidine, the structure isn’t just an academic puzzle. It unlocks experiments that track molecules in living systems, or design drugs with fewer unwanted effects. Researchers dig into NMR, mass spectrometry, and X-ray crystallography for a reason—every atom’s place becomes a clue to activity, safety, and use.
Most people don’t stare at chemical diagrams after breakfast, yet the structure of a single molecule shapes entire industries. Understanding complex heterocycles and their modifications makes drug discovery faster, cheaper, and more rational. For this compound, the iodine atom could open doors in cancer imaging or specialty syntheses. By focusing on structural details, researchers avoid guesswork and point efforts toward success. Investment in training, advanced analytical tools, and organic synthesis will push the usefulness of these molecules further, putting science a step ahead of disease and environmental challenges. The details in chemical structure matter—for scientific progress, for patient outcomes, and for a healthier world.
4-(4-Iodo-1H-pyrazol-1-yl)piperidine isn’t a household name. In lab circles, though, it sparks recognition. Tucked behind this long chemical name sits a structural component that drives much of the day-to-day innovation in drug discovery and chemical biology. With experience on both the research and industry sides, I’ve watched this class of molecule move quietly into projects that touch everything from cancer therapy to basic neuroscience research.
Chemical scaffolds aren’t just random bits stuck together—they shape the way drug candidates interact with targets in our bodies. Medicinal chemists use compounds like 4-(4-Iodo-1H-pyrazol-1-yl)piperidine to design molecules that plug into specific enzymes or receptors. That iodine atom sticking out adds a particular heft, helping researchers track where the molecule goes or dial up its potency.
Big names in pharma have spent decades searching for enzyme inhibitors or receptor antagonists using similar core structures. It’s common in kinase research, as kinases play starring roles in cancer, inflammation, and metabolic diseases. The pyrazole and piperidine portions don’t just nail down activity; they help with molecular tuning, making the compound more or less sticky to its target. Some of the most promising kinase inhibitors and CNS (central nervous system) drugs built up their profiles using tweaks to these kinds of scaffolds.
Scientists often need to know more than what binds—they need to know how and where. The heavy iodine atom serves almost as a chemical marker. Iodinated compounds work well in radioactive labeling, such as with iodine-125, so researchers can track the movement of the compound inside a cell or animal. Watching where these tagged molecules land helps scientists map out biological pathways in a way that’s tough to match with untethered compounds.
I worked with a research team using radioiodinated probes to target mutant enzymes in cancer cells. Using an analogue of this compound, we traced the compound’s journey from injection to accumulation in tumors. The clarity it brought to our animal imaging studies saved weeks of guesswork and refined dosing for therapies later aimed at people.
Beyond the direct hunt for new medicines, chemists lean on such compounds for synthetic transformations. The aryl iodide handle acts as a launch point for Suzuki coupling and other modern reactions. Chemists can snap on different functional groups where the iodine sits, rapidly making new molecules for screening. This modular approach lets research teams pivot quickly based on early testing results, shifting gears without scrapping months of prior work.
Restricted access due to regulatory barriers or supply chain slowdowns can bottleneck labs. Sourcing high-purity material isn’t just about cost, but reliability and safety in use. Some labs work with local chemical vendors or set up in-house synthesis protocols to avoid delays. Others pool resources across university departments to ensure critical intermediates don’t dry up mid-project. Transparency in sourcing—provenance of chemicals, purity, and storage practices—helps researchers trust their results and reduces variability, long before any findings enter clinical trials.
There’s a certain satisfaction in realizing how small tweaks in molecules, like the addition of a single iodine atom, can move whole fields forward. Real value comes in the hands of patient scientists who don’t just chase molecules for their novelty, but for the specific questions they answer. That approach, over decades, is how basic research blooms into genuinely new therapies or clearer models of disease.
I’ve spent a good chunk of my chemistry career managing sensitive reagents. Watching people in a rush cut corners with storage taught me early why some rules exist. 4-(4-Iodo-1H-Pyrazol-1-Yl)Piperidine won’t explode on your shelf, but ignore its needs and you risk wasted resources, lab downtime, and possibly damaged experiments down the line.
Iodinated compounds share one trait: they dislike moisture. Leave this molecule in a humid room and you might find it clumped up, even degraded. I keep mine locked away in a tightly sealed amber glass bottle, with a couple of silica gel packets for good measure. Humidity above 60% in storage rooms starts trouble you won’t see until the next thin-layer chromatography run shows extra spots.
Light sneaks up as another problem. Pyrazole rings, especially with halogen attachments, don’t like direct sunlight or strong indoor bulbs. In my lab, anything with an iodo tag goes in a drawer or, for more volatile samples, wrapped in aluminum foil. It’s a simple thing that spares you from surprise decomposition, especially after a weekend under the bench lights.
People trust room temperature storage for many chemicals. Yet, 4-(4-Iodo-1H-Pyrazol-1-Yl)Piperidine lasts longer below 8°C. In my experience, putting it in a laboratory fridge (not the freezer, as frost can add its own moisture) adds months to its reliable shelf life. At ultra-low temps, some pyrazole derivatives crystallize oddly, so I avoid deep freezing unless the manufacturer’s datasheet specifically endorses it.
With dozens of small bottles crowding any chemical storage cabinet, clear labeling makes a difference. Permanent marker wears off, so I use solvent-resistant labels. Since iodine-containing organics sometimes interact with strong bases or oxidizers nearby, I don’t shelve them together. In my last position, an unmarked bottle ended up beside an oxidizer—fortunately, nothing happened, but the potential for danger was real. Keeping reactive materials apart isn’t busywork—it’s common sense learned from close calls.
Every manufacturer I’ve ordered from gives guidance on storage, but not all supply thorough details. PubChem and the European Chemicals Agency lay out similar advice: dry, dark, cool conditions, avoid contact with oxidizers and bases. If you’re reading a safety data sheet that simply says “Store at room temperature,” take extra steps if your lab has high humidity or fluctuating temperatures. Document what you’re doing—future lab members will thank you for that act of care.
Good chemical storage isn’t rocket science. Dry environment, steady low temperature, and protection from light do most of the heavy lifting. Small steps shave hours off troubleshooting failed syntheses. In my experience, investing five minutes up front for proper storage beats remaking everything from scratch. And if you’re ever unsure, ask the supplier or consult a seasoned chemist. They’ve probably had to clean up after a storage mishap at least once themselves.
People in chemical research often talk about specialty intermediates. 4-(4-Iodo-1H-Pyrazol-1-Yl)Piperidine stands out in conversations about advanced pharmaceutical building blocks because of its core structure. Its molecular shape helps researchers for drug discovery, especially in areas like oncology and neuroscience. Still, outside a few focused labs and pilot plants, most professionals never see this compound sold by the drum or in hundred-kilo orders. There is a reason why bulk buyers keep running into headaches.
Ask a procurement manager in pharmaceuticals about sourcing something like this molecule, and they’ll tell you the market often feels like a closed club. Large chemical distributors list broad catalogs online, but specialized intermediates tend to require direct negotiation. Price is only part of the puzzle. Lead times stretch for months. You still need strong paperwork, import permits, and confidence in supply chain integrity. Labs struggle to keep stocks steady on their shelves, and scale-up becomes risky without a dependable contract manufacturer. Smaller vendors on international trading platforms sometimes offer grams or tens of grams, but these quantities seldom meet the thresholds for a commercial process.
Chemical routes for producing 4-(4-Iodo-1H-Pyrazol-1-Yl)Piperidine are tricky. Reactions may involve delicate steps like iodination and pyrazole coupling. Each batch brings risk of impurities, batch variability, and waste management issues. Manufacturers hesitate to pledge ongoing bulk production for a relatively niche target over cheaper, higher-volume commodities. Safety and regulatory hurdles add costs and discourage overproduction, especially as global oversight tightens around specialty chemicals with toxic or hazardous precursors.
Pharmaceutical and biotech companies want to lower costs but not at the expense of quality or compliance. Reputable suppliers with documented quality systems often restrict larger sales unless buyers prove they meet regulatory standards. This helps block misuse or diversion of chemical intermediates into illegitimate hands. In my own work with supply chain audits, I’ve seen that transparency and traceability remain top priorities—buyers must check not only paperwork but also site certifications and the presence of experienced chemists on staff. Sometimes, the lack of bulk inventory stems from business decisions to safeguard intellectual property and minimize risk exposure.
Chemists and supply managers can take steps to avoid shortages and delays. Forming long-term arrangements with reputable custom synthesis providers, who hold proven experience with halogenated pyrazole chemistry, creates breathing room for project timelines. Buyers often have better luck with suppliers rooted in regions known for specialty chemicals—parts of Europe, Japan, and contract manufacturers in the United States. Transparent, proactive communication helps clarify required purity and documentation early, making it easier for a supplier to commit to a larger-scale run. Promoting open networks for sourcing, using trade associations, and supporting collective buying can motivate producers to stock bulk inventory.
Shortages and unpredictability in the global chemical market won’t disappear overnight, but steady, informed demand and closer relationships between chemists, quality reviewers, and producers can make sourcing less of a guessing game for important intermediates like 4-(4-Iodo-1H-Pyrazol-1-Yl)Piperidine.
In chemical synthesis, numbers say a lot about commitment to quality. For a product like 4-(4-Iodo-1H-Pyrazol-1-Yl)Piperidine, purity often lands right at the top of the checklist for anyone working in research, pharmaceuticals, or complex organic chemistry. Companies confident in their synthetic know-how set purity at above 98%, and that commitment makes a genuine difference.
Think about what happens if you skip out on purity standards. Impurities create side reactions that lead researchers down frustrating dead ends. There’s a reason why medicinal chemists, the people formulating the next lifesaving treatments, place purity on equal footing with reproducibility. From my own experience in a small-scale research lab, I recall how ambiguous results can send project timelines off course. One batch of poorly characterized intermediate cost us three weeks and a chunk of funding.
Data from the American Chemical Society underscores the same: even a 1% gap in purity can shrink yields, complicate separations, and cast shadows over analytical results. This is not theory—these are practical obstacles that waste resources and erode confidence.
Lab teams demand more than just a label. Standard chromatograms, NMR, LCMS, and HPLC reports build trust between supplier and end user. Full transparency in paperwork, looking at the residual solvents, trace metals, or even water content, lets people judge the compound with a critical eye. My experience tells me, when a supplier delivers complete documentation, troubleshooting during synthesis simply drops.
Huge discrepancies typically show up when analytical results fail to match bold claims. I’ve watched experienced chemists quickly catch questionable purity through basic TLC and melting point data. Any company that performs proficiency checks and supports data with certificates sets a mark above just “meeting specification.”
Getting a bottle of 4-(4-Iodo-1H-Pyrazol-1-Yl)Piperidine that meets a 98% or higher purity means reactions produce what chemists expect. This reduces time spent hunting unknown byproducts. At my last position, each impurity took us hours to track, and left everyone staring at unexplainable NMR peaks. Consistency in purity does more than help with the next batch; it means published results reflect what really happened, raising trust across the scientific community.
Strict quality checks start before a product leaves the supplier. Good companies back up purity claims with both in-house and third-party validation. No chemist has time for surprises hidden in a bottle. Processes like high-resolution mass spectrometry, potassium iodide tests for residual iodine, and regular cross-batch sampling keep everyone honest.
Sometimes a certificate gets stapled on and forgotten. Yet the next level of trust comes from suppliers who invite open questions, accept returns when things go wrong, or share information about how and where batches are tested. If someone asks about chiral purity or unknown minor peaks in the spectra, prompt answers show real respect for the discipline.
Product stewardship grows from open communication, not just a number printed on a vial. For those of us in fast-paced labs, every line on a spec sheet represents time and money saved. Sometimes, everything comes down to that 98%—and the confidence that a product lives up to its claim.
| Names | |
| Preferred IUPAC name | 4-(4-iodopyrazol-1-yl)piperidine |
| Other names |
PubChem23124335 SCHEMBL10602855 |
| Pronunciation | /ˈfɔːr fɔːr ˈaɪədoʊ wʌn eɪtʃ paɪˈræzɒl wʌn aɪl paɪpəˈrɪdiːn/ |
| Identifiers | |
| CAS Number | 907194-10-5 |
| 3D model (JSmol) | 3D model (JSmol) string for **4-(4-Iodo-1H-Pyrazol-1-Yl)Piperidine**: ``` CC1CCN(CC1)N2C=CN=C2I ``` *(Note: This is the **SMILES** string representing the molecular structure, which is used by JSmol for 3D visualization.)* |
| Beilstein Reference | 5952272 |
| ChEBI | CHEBI:137806 |
| ChEMBL | CHEMBL3753401 |
| ChemSpider | 22386682 |
| DrugBank | DB08302 |
| ECHA InfoCard | 04f1d8a1-0133-41e0-b41f-177a960c3d6e |
| EC Number | 1005582-23-1 |
| Gmelin Reference | Gmelin 841651 |
| KEGG | C19385 |
| MeSH | 4-(4-Iodo-1H-Pyrazol-1-Yl)Piperidine" does not have a corresponding MeSH (Medical Subject Headings) term as of now. |
| PubChem CID | 166634302 |
| RTECS number | UJ6ZL2WK6G |
| UNII | X6R8C9X4PJ |
| UN number | UN3276 |
| CompTox Dashboard (EPA) | DTXSID5022098 |
| Properties | |
| Chemical formula | C8H12IN3 |
| Molar mass | 358.17 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.78 g/cm³ |
| Solubility in water | Insoluble |
| log P | 1.28 |
| Acidity (pKa) | pKa = 11.15 |
| Basicity (pKb) | 4.53 |
| Refractive index (nD) | 1.674 |
| Dipole moment | 2.77 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 380.6 J·mol⁻¹·K⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | CC1CCN(N=C1)C2=CN(N=C2)I |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| NIOSH | QN9DJ10KYR |
| PEL (Permissible) | No PEL established. |
| REL (Recommended) | 10 mg |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
4-(1H-Pyrazol-1-yl)piperidine 4-(4-Bromo-1H-pyrazol-1-yl)piperidine 4-(3-Iodo-1H-pyrazol-1-yl)piperidine 4-(4-Iodo-1-methyl-1H-pyrazol-1-yl)piperidine 4-(4-Chloro-1H-pyrazol-1-yl)piperidine 4-(4-Fluoro-1H-pyrazol-1-yl)piperidine |