Chemists have chased after piperidine derivatives for decades, drawn in by a mix of curiosity and practical need. 4,4-Dimethoxypiperidine represents a leap taken in the late 20th century, when folks in pharmaceutical labs realized the potential hiding in piperidine’s skeleton. At the start, the goal often circled around building blocks for more complex organic frameworks. Over the years, as demand for new heterocyclic compounds blossomed, methods for synthesizing and applying dimethoxypiperidines sharpened. Lab notebooks from both academic and industrial settings roll out a timeline, one that shows chemists learning from trial, error, and technological advances in purification and analytics. These lessons—plus tighter regulations and a push for greener chemistry—shaped the chemical’s use in research and product development.
4,4-Dimethoxypiperidine carries the weight of its niche role in organic synthesis and pharmaceutical design. You won’t find it on a pharmacy shelf, but walk into a research lab and odds lean toward its presence as a starting point, reagent, or intermediate. Its structure—the piperidine ring dressed up with two methoxy groups at the 4-position—helps the molecule stand out when compared to other ring systems. Labs that crank out small molecules for pharmaceutical screening or specialty materials often keep it stocked for the straightforward way it can unlock new chemical spaces.
Let’s get to brass tacks: at room temperature, this compound shows up as a clear liquid or a crystalline solid, depending on how it’s handled and stored. Its molecular formula, C7H15NO2, puts its molar mass close to 145.2 g/mol. The methoxy groups at the 4-position shield certain reactions but open the door to others, letting 4,4-dimethoxypiperidine walk the line between stability and reactivity. The piperidine core naturally boosts the basicity and electron richness, so the compound plays well with a range of nucleophiles and electrophiles. Its moderate boiling point helps during distillations, while solubility in common organic solvents like dichloromethane or acetone really makes it flexible for synthetic routes.
Sourcing high-purity 4,4-dimethoxypiperidine usually means getting a product at or above 98% purity. Sometimes labs care even more about moisture and residual solvents, as trace contaminants can throw off catalytic reactions or mess with analytical signals. Labs keep a sharp eye on labeling: safety warnings about toxicity, flammability, and storage requirements must show up clearly. Suppliers list shelf life, CAS number, recommended storage temperatures, and incompatibles—think strong acids or oxidizers—since mishandling can lead to spoilage or hazardous events. Barcode tracking and digital inventory systems now support compliance with regulatory traceability standards.
Synthesizing 4,4-dimethoxypiperidine usually starts with piperidine or its 4-substituted derivatives. One common method uses 4-piperidone as a precursor, reacting it with methanol under acid catalysis. Methoxylation at the 4-position happens with formaldehyde and a methanol soak, producing that signature dimethoxy appearance. Labs prefer routes that crank out fewer side products, since separating diastereomers or tarring out on columns eats time and money. Reaction conditions key in on moderate temperatures and controlled additions of acid, balancing yield and selectivity. I remember trying a similar reaction as a student; making adjustments for humidity and glassware cleanliness often meant the difference between a clean product and a gunky mess.
4,4-Dimethoxypiperidine doesn’t just sit on a shelf. The methoxy groups at the 4-position tempt chemists to tweak and swap, converting them to hydroxyls, amines, or other substituents. Reductive demethylation—using iodocyclohexane or Lewis acids—lets researchers strip off the methoxy protection and unmask functional handles for further elaboration. Chemists aiming at library synthesis find the molecule's ring scaf-fold—especially when blended with other reactive groups—useful for laying down a framework on which medicinal chemists can hang all sorts of new groups. Alkylation and acylation at the nitrogen atom let it branch off in new structural directions. Its entire profile encourages exploration, and that sense of possibility keeps researchers coming back.
A scientist or technician may call it 4,4-dimethoxypiperidine, but databases keep alternate monikers at the ready—4,4-DMP, N-methyl-4,4-dimethoxypiperidine, or just DMP for shorthand. Chemical catalogs don't just push one name; they cycle through variations, reflecting both supplier preferences and regional standards. This name swapping creates occasional confusion, especially in multinational projects. Modern inventory tools and properly cross-referenced databases help labs avoid costly mix-ups that could derail days of painstaking work.
Handling 4,4-dimethoxypiperidine asks for a clear safety protocol. The compound can irritate skin, eyes, or lungs, so gloves, goggles, and sometimes a fume hood answer as non-negotiable. Spills require absorbent pads, and waste must roll through proper disposal streams—hydrolysis can decompose it but, if tossed carelessly, toxic byproducts risk entry into water systems. Transport sticks to legal thresholds for hazardous chemicals. Training for new users often ties in explanations about accidental exposure, route of entry, and effective first aid. I’ve seen accidents happen when folks underestimate these 'small molecule' hazards; it only takes one careless moment to land in a health clinic or needing environmental remediation.
The reach of 4,4-dimethoxypiperidine stretches wide, though its highest impact shows up in the early stages of pharmaceutical synthesis. Drug designers use it to scaffold potential new treatments—modifying its ring, building out new analogs, and testing activity at a slew of biological targets. Materials chemists sometimes reach for it when aiming to adjust polymers or add flexibility and stability to new classes of plastics. Research efforts exploring agrochemicals, dyes, and specialty coatings see periodic interest in this compound. It even turns up in mechanistic organic studies, where its methoxy groups let chemists probe reactivity trends and ring conformations.
Refining synthetic routes and expanding the toolkit for modifying dimethoxypiperidine leads research priorities today. Teams push for greener, more atom-efficient reactions that minimize waste. Automated synthesis techniques and machine learning offer a fresh lens on process optimization, sometimes uncovering unexpected reactivity or simplified purification approaches. Focus on chiral forms—handedness at the atomic level—also lands here, especially when tailoring compounds for pharmacological or catalyst purposes. Current literature buzzes about structure-activity relationships, hoping one tweak could unlock new activity against diseases or open up a shortcut in total synthesis. In my own experience, cross-disciplinary work—chemists, data scientists, and analytical experts—almost always uncovers unexpected pathways that simplify how we access these molecules.
4,4-Dimethoxypiperidine draws careful scrutiny from toxicologists. Tests show the compound can deliver mild to moderate irritation, but the real questions circle long-term exposure or metabolic breakdown. Research continues on whether its methoxy groups offer extra protection or unexpected bioactivity—animal studies so far suggest careful monitoring should guide any application that brings the substance into direct human contact. Many labs screen for mutagenicity, genotoxicity, and environmental persistence, because rules get stricter every year. Continuing collaboration with regulatory bodies ensures new uses don’t outpace our understanding of potential risks. As someone who once worked on a project halted by late-breaking toxicology results, I can say that early and thorough investigations pay off in peace of mind, slower costs, and safer products.
The arc ahead for 4,4-dimethoxypiperidine ties back to the broader trends in green chemistry, targeted synthesis, and the hunt for next-generation pharmaceuticals. Researchers push to design more selective reactions, trim down the need for protecting groups, and reach for sustainable solvents and catalysts. Digital modeling and high-throughput experimentation accelerate the search for structurally related compounds with new activity. Regulatory winds blow toward greater transparency and detailed safety profiles, and those pressures shape both manufacturing and innovation. As industries adapt and the global community calls for responsible chemical management, the story of this molecule, along with its peers, weaves into the bigger picture—one that values creativity, safety, and teamwork at each step.
4,4-Dimethoxypiperidine doesn’t exactly roll off the tongue, but this organic compound has real value in labs around the world. Its main appeal comes from a simple piperidine core, dressed up with two methoxy groups at the fourth carbon. Chemists recognize the impact those methoxy groups bring, as they tweak the molecule's reactivity and open new opportunities for making other substances.
My early days in the lab involved plenty of catalog-reading and chemical prep. I learned quickly that certain building blocks, like piperidine derivatives, crop up often in pharmaceutical research. 4,4-Dimethoxypiperidine stands out in this group. With its structure, chemists craft new molecules more efficiently, especially those aimed at treating neurological conditions or managing pain.
The earliest thing to know about 4,4-Dimethoxypiperidine is its status as an intermediate. Many modern drug candidates depend on reliable intermediates to reach the finish line. Medicinal chemists often reach for this piperidine to help assemble complex drug structures. It shows up in routes leading to antipsychotics, antidepressants, and other central nervous system agents. The presence of two methoxy groups shapes its reactivity, which lets custom syntheses happen with fewer steps and fewer pitfalls.
Research papers and patents highlight this pattern over and over. For instance, some studies link 4,4-Dimethoxypiperidine to the manufacture of compounds related to atomoxetine—a treatment for attention-deficit hyperactivity disorder (ADHD). Knowing that, it’s easy to see why labs stock up despite the niche name.
No chemical enters the research or commercial supply chain without some risk. 4,4-Dimethoxypiperidine requires careful handling. It raises little debate as a precursor for designer drugs. A quick look at regulatory data shows that a few countries treat it with extra scrutiny. I’ve chatted with regulatory officers who point out how law enforcement sometimes sweeps up piperidine derivatives during investigations into illegal labs.
Proper supply chain management and secure handling go hand-in-hand with ethical practice. Reputable chemical suppliers verify their buyers. This works as a frontline defense against misuse. Most researchers have no reason to bend the rules, but the checks are always there. Some labs keep logs of who accesses such compounds and why, knowing that trust builds with transparency.
Science asks for open exchange of ideas and materials, but the wider community also expects responsibility. One thing I find useful: clear communication between researchers, suppliers, and regulators. This keeps everyone honest and helps early detection if a harmful trend emerges. Training new scientists means teaching lab safety, and it also means teaching ethics. If someone asks about 4,4-Dimethoxypiperidine and its use, the real answer goes beyond just a chemical equation or a reaction yield. It’s about what we’re building, how we use our tools, and who benefits at the end of the day.
4,4-Dimethoxypiperidine serves a role that sits in both the spotlight and the shadows. It enables progress in medicine but also calls for old-fashioned vigilance and responsible decision-making. That’s the reality for many tools in modern science.
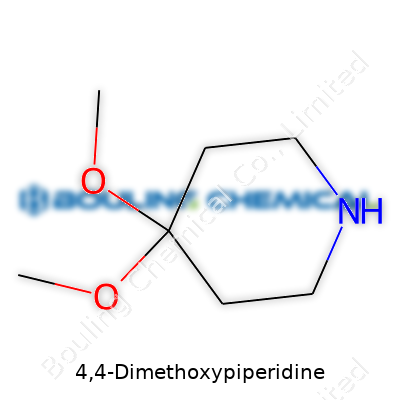
Piperidines pop up everywhere in the world of chemistry, from the backbone of drug discovery to the base of chemical synthesis. Among them, 4,4-Dimethoxypiperidine holds its own with a structure easy to recognize: a six-membered piperidine ring topped off by two methoxy groups pointing out from the same carbon at the fourth spot. A chemist picturing this ring can’t help but think of the classic nitrogen-containing hexagon, a true building block for so many molecular innovations.
The backbone here, that piperidine ring, stars one nitrogen placed in a six-membered ring of carbons. At the fourth position around the ring, two methoxy groups (that’s an oxygen and a methyl, or CH3O-) anchor in, taking up space and shaping the ring’s properties in clear ways. Anyone with organic chemistry under their belt recognizes the value methoxy groups add, changing how the molecule acts and how it reacts with other compounds.
Talking shop, the molecular formula of 4,4-Dimethoxypiperidine looks like this: C7H15NO2. Imagine taking a piperidine and simply swapping two hydrogens at the fourth position with methoxy groups. The carbon skeleton of that ring doesn’t get complicated, but those additions totally reshape its reactivity. No need for advanced visualization tools to see how two oxygen atoms and their methyl branches adjust polarity, solubility, and metabolic behavior. These things help a chemist predict what comes next in a reaction.
I’ve spent enough time watching junior chemists marvel at how a little tweak in a ring system can either block or encourage a reaction. Here, the methoxy groups shield the fourth position of the piperidine, protecting it from easy reactions that might happen if there were just hydrogens. This kind of steric hindrance comes in handy if someone plans to build something complex on top of the ring without risking over-reactions at unwanted spots.
These added functional groups tweak basicity too, affecting how this ring interacts with acids, bases, and metal catalysts. In the real world, that helps guide a reaction or protect a molecule when the time comes to pull off another transformation. Medicinal chemists often favor groups like this for tweaking how a drug molecule fits a target or moves through the body, since small changes can feed big differences in absorption or metabolism.
This is not just a theoretical molecule for textbooks. 4,4-Dimethoxypiperidine plays key roles in intermediate steps for pharmaceuticals and advanced materials. The piperidine ring is a favorite for its stability and the ease of modifying it. Companies use molecules like this for creating scaffolds in drug candidates or adding protective layers to pieces of larger synthetic puzzles. I’ve seen research move from frustrating dead ends to real progress just by swapping in a methoxy-protected ring at the right moment.
One sticking point comes up during mass production. Those methoxy groups, useful as they are, sometimes make purification a tough job. It takes careful workup to avoid leftover by-products or over-reaction. Clear, stepwise reaction planning and modern purification—crystallization, chromatography—stay essential tools. When unexpected side reactions pop up, extra vigilance in monitoring and tweaking the reaction conditions helps smooth out the process.
By paying close attention to the chemical structure of 4,4-Dimethoxypiperidine and its unique properties, researchers keep finding new ways to use this compound in building more effective drugs, better materials, and even new synthetic techniques. Advances in chemistry grow out of these kinds of smart modifications—each group, each tweak, bringing fresh tools to the science bench.
4,4-Dimethoxypiperidine isn’t a chemical you’ll come across at the pharmacy or in household items. Labs and chemical manufacturers use it to build more complex compounds, mostly in research settings. Seeing an unfamiliar chemical name gets people thinking about safety and toxicity, which is fair, since chemical mishaps rarely end well for people caught off guard.
Getting hands on proper safety information means looking at material safety data sheets. For 4,4-Dimethoxypiperidine, you don’t see pages of horror stories, but it’s not marked as harmless either. Many alkyl piperidines bring up risks of skin, eye, and respiratory irritation. If it lands on your skin, expect discomfort. Accidentally breathe in fine powder or vapor, and your lungs might sting badly. No, it doesn’t carry the worst danger labels, but any lab chemist wearing gloves and goggles around it isn’t being paranoid.
Digging through toxicology references, you won’t find mountains of research. There isn’t a long list of animal studies or human exposure incidents to give an exact toxic dose. With chemicals like this, the unknown is nearly as serious as proven hazards. Similar compounds can trigger nervous system effects at higher doses. A lab can estimate the risk but never call it completely safe without testing.
Reports on related compounds show issues occur more with chronic (long-term, repeated) exposure rather than brief, one-off encounters. Many workers handle possible irritant chemicals for years and only develop symptoms after a lot of contact. Dermatitis kicks in if bare skin keeps getting splashed. Eyes can become sensitive or inflamed from repeated exposure to vapors or dust, so prevention always beats cure.
Sitting in a lab or handling unknown chemicals always put safety front and center. Once, as a chemist myself, I ended up with a mild chemical burn just because I skipped gloves for "just a quick pour." The stinging lasted hours. Folks learn fast in these places: taking shortcuts means inviting problems. Respirators, gloves, eye protection—these aren’t overkill. Industrial and academic guidelines reflect the inconvenience and pain that result when someone tries to handle raw chemicals with bare hands or no mask.
Waste disposal counts as another piece of the puzzle. Labs and waste handlers don’t just pour unknown chemicals down the drain. Local regulations demand safe disposal, protecting not just the worker but water supplies and the neighborhood nearby. Nobody wants mysterious substances accumulating downstream.
Keep training new staff. Go over chemical hygiene plans. Insist on personal protective equipment every time. Lock hazardous materials up tight. In the broader picture, industry and academia both need to keep evaluating chemicals for hidden long-term effects, not just acute hazards or legal minimums.
If it leads to discomfort, burns, or organ damage after years of small doses, everyone deserves to know and adjust protocols. Making chemical work safer never ends. Real progress means sharing lessons, updating data, and giving people the right tools and training to protect themselves every time they handle a bottle or beaker.
4,4-Dimethoxypiperidine, a chemical recognized for its role in organic synthesis and pharmaceutical building blocks, makes its way through labs thanks to its flexibility. I’ve worked in academic and contract research spaces, and anyone handling this compound needs to pay attention to its chemical personality to avoid headaches later.
Think of this compound as a somewhat sensitive guest: not fast to react, but not immune to neglect. At room temperature, 4,4-Dimethoxypiperidine usually appears as a colorless liquid or sometimes a faint solid depending on purity and storage conditions. Things can go wrong if temperatures go soaring or tanks chill far too low, so the right balance is important.
Small details around storing chemicals have big knock-on effects. For 4,4-Dimethoxypiperidine, neglecting temperature or sealing can wreck both results and safety. I’ve seen seasoned coworkers lose material value because someone ignored common-sense rules. Safety data sheets describe how this piperidine derivative doesn’t love excessive heat or open air. Fluctuating temperatures risk bottle degradation or that funky, irritating smell that signals chemical decomposition.
Every time I’ve ordered new stock, the best suppliers pack containers tightly and clearly mark expiration dates. Any lapse in dryness or temperature spikes brings risk, so letting bottles linger in humid or sunlight-filled corners isn’t smart practice.
Safe storage begins with a tightly sealed, corrosion-resistant bottle—usually amber glass works fine, especially if your bench gets hit with sunlight. I’ve always found desiccators or ventilated cabinets effective, because they keep humidity out and light to a minimum.
Storing this material in a cool spot—think general lab ambient, away from radiators or hotplates—makes the most sense. Standard recommendation puts the sweet spot between 2°C and 8°C, which is regular fridge territory. Pharmaceutical research often includes cold rooms precisely for chemicals like this. Direct light can speed up breakdown, so an opaque or amber-colored container goes a long way.
Other key pointers: don’t trust reused plastic containers, avoid jumbled shelves that risk spills, and keep away from acids or oxidizers. I’ve watched accidents happen when incompatible chemicals found each other in a cluttered cabinet. Stacking similar bottles together (piperidines with other amines) and adding clear hazard labels cuts down on mistakes.
Goggles, nitrile gloves, and good ventilation always matter, but long-term safety includes a solid inventory and regular checks. I learned to log bottle arrival and opening dates, which catches degradation issues early. Trusted lab environments train new staff to avoid transferring small amounts to secondary containers unless they get relabeled and resealed right away.
Leaks don’t just waste money—they also trigger hazardous fumes and fire risks. Absorbent pads or secondary containment bins add a helpful layer of insurance against tipping and accidental breakage.
Many labs hit snags during summer heat waves or facility shutdowns. I’ve seen cooling failures ruin entire stocks in a single weekend. Lab managers like to install backup power on refrigerators and post routine inspection checklists by cabinets where heat and humidity can sneak in.
Every lab should review local regulations and manufacturer instructions, because some storage differences arise based on local climate or chemical scale. As a group, chemists and lab techs share tips on forums or group chats—someone's experience with a mishap usually turns into a good protocol update for everyone else. Lessons learned from honest mistakes keep safety culture alive.
Experienced chemists know shortcuts on storage rarely end well. With 4,4-Dimethoxypiperidine, paying attention to basic storage from the first day in the lab protects research quality, personal health, and those who’ll work in the space after you. Respecting these simple steps keeps lab life running smoothly—and keeps this useful reagent reliable for the next set of experiments.
Ask any researcher: buying reagents takes more than a few clicks. Walk into a university lab and you’ll find the molecular shopping list tacked above the workspace. On it, novel intermediates bump shoulders with classic solvents, but complicated molecules like 4,4-Dimethoxypiperidine always trigger extra scrutiny.
This compound often pops up in pharmaceutical research and organic synthesis. Chemists use it as a building block to create new molecules, targeting treatment for neurological disorders, or devising new agrochemicals. People who don’t work behind a bench may never cross its path. On the other side, for someone designing new reactions, this piperidine derivative helps unlock new possibilities and pathways.
If you’re gearing up to place an order, be prepared for paperwork. Online suppliers like Sigma-Aldrich, Alfa Aesar, and TCI Chemical stock this compound, but they don’t ship it like a set of headphones. My first time sourcing a niche molecule, I sent in institutional credentials and project details for a safety check. Even years later, most suppliers still want to see proof of lab affiliation, business credentials, intended use, and local permits. This protects both the buyer and supplier, especially since substances like 4,4-Dimethoxypiperidine have uses beyond routine chemistry. Regulators keep a close eye on shipments due to risks tied to chemical diversion and misuse in illegal synthesis.
Legitimate purchases start with reputable suppliers. Sigma-Aldrich and Fisher Scientific both require business or research registration—no retail checkout for home experimenters. Their vetting process cuts down on accidents and illegal activities. This level of precaution means fewer stories of mishandled chemicals in the news and more research breakthroughs in journals.
Smaller specialty vendors like Oakwood Chemical or Acros Organics sometimes carry stock, but they also stick to strict due diligence. Buy from anyone offering lax policies and you invite counterfeit chemicals or, worse, brush up against law enforcement. A wise move: check for certifications, customer reviews, and country-specific import/export regulations. China and India supply a lot of these chemicals, but international buyers face customs hurdles, shipping delays, or outright bans if papers aren’t complete.
Tough rules may leave underfunded researchers in a bind, but safety trumps convenience. In my lab days, ordering controlled substances slowed budgets and timelines, yet nobody seriously wanted to see dangerous items land in the wrong storage locker or garage.
One solution looks like better partnerships: universities and small start-ups can team up with bigger labs to negotiate group orders, share compliance resources, and pool storage facilities. Clear government guidelines that spell out requirements go a long way too. When I taught new chemists, walking through the procurement process became an education in itself, teaching the real-world cost of carelessness.
Finding a reliable source for 4,4-Dimethoxypiperidine means navigating regulations, vetting suppliers, and demonstrating need. This creates guardrails that support not just research, but also public safety. Lifting the veil on these behind-the-scenes challenges makes it easier for scientists, suppliers, and regulators to work together on responsible progress.
| Names | |
| Preferred IUPAC name | 4,4-Dimethoxypiperidine |
| Other names |
NSC 69969 4,4-Dimethoxy-1-piperidine 4,4-Bis(methoxy)piperidine |
| Pronunciation | /ˈfɔːr fɔːr daɪˌmiːˈθɒksi pɪˈpɛrɪdiːn/ |
| Identifiers | |
| CAS Number | 40444-65-7 |
| Beilstein Reference | 604468 |
| ChEBI | CHEBI:69585 |
| ChEMBL | CHEMBL3225951 |
| ChemSpider | 5310810 |
| DrugBank | DB08385 |
| ECHA InfoCard | ECHA InfoCard: 100_011_763 |
| EC Number | 219-026-8 |
| Gmelin Reference | 89079 |
| KEGG | C18711 |
| MeSH | D011614 |
| PubChem CID | 118444 |
| RTECS number | TZ4600000 |
| UNII | T8O4922E8N |
| UN number | UN2810 |
| CompTox Dashboard (EPA) | 4,4-Dimethoxypiperidine CompTox Dashboard (EPA): "DTXSID70116461 |
| Properties | |
| Chemical formula | C7H15NO2 |
| Molar mass | 161.23 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Odorless |
| Density | 1.06 g/cm3 |
| Solubility in water | soluble |
| log P | 0.03 |
| Vapor pressure | 0.27 mmHg (at 25 °C) |
| Acidity (pKa) | pKa = 11.2 |
| Basicity (pKb) | 2.81 |
| Magnetic susceptibility (χ) | -66.8e-6 cm³/mol |
| Refractive index (nD) | 1.480 |
| Viscosity | Viscosity: 0.948 cP (25°C) |
| Dipole moment | 2.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 234.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -3913.7 kJ/mol |
| Hazards | |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. |
| Precautionary statements | P261, P264, P271, P272, P302+P352, P321, P363, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 74 °C |
| NIOSH | NA |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 ppm (40 mg/m3) |
| IDLH (Immediate danger) | NIOSH has not established an IDLH for 4,4-Dimethoxypiperidine. |
| Related compounds | |
| Related compounds |
Piperidine 2,6-Dimethylpiperidine 4-Methoxypiperidine N-Methylpiperidine 4-Piperidone |