Researchers have been tinkering with piperidine rings for decades, but the difluorinated version, 4,4-difluoropiperidine, made its mark as synthetic chemistry leaned harder into the world of fluorine. The appetite for new ways to incorporate fluorinated motifs picked up in the late 20th century, as folks noticed these tweaks often improved pharmacological behavior. Laboratories discovered that dropping two fluorines onto the 4-position of a classic piperidine skeleton produced something unique, something with both stability and fresh chemical possibility. Over the years, chemists pushed this compound into the spotlight for its ability to serve as a backbone in various syntheses, especially in the pharmaceutical patchwork.
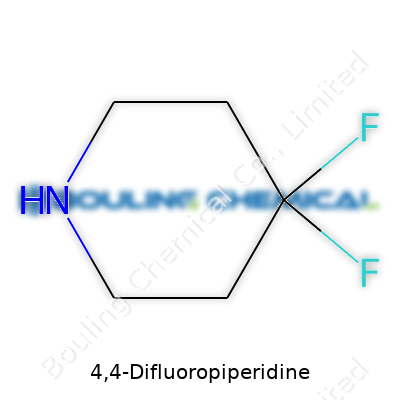
4,4-Difluoropiperidine stands out from the standard toolkit of piperidine derivatives because it brings fluorines into play without throwing off the basic structure. A six-membered ring of five carbons and one nitrogen, this molecule wears two fluorine atoms at the 4-slot, and that little tweak gives it added stability along with altered electronic properties. Chemists pick up this compound in search of something more robust or looking for a handle to attach other functional groups, and the subtle differences introduced by the fluorines become the make-or-break point for certain drug candidates or specialty materials.
If you crack open a bottle in the lab, you’re likely to find a colorless liquid or a low-melting solid, depending on how dry and pure it is. Fluorination here tightens up the reactivity, with the bond angles near the difluorinated carbon tweaked compared to regular piperidine. Its boiling point sits higher than you’d see with the unsubstituted ring, and the participation of fluorine tends to reduce basicity at the nitrogen—important if you’re trying to attach a new group or pick out a reaction’s next step. 4,4-difluoropiperidine resists metabolic breakdown better than its hydrogen-rich sibling, which matters when making drugs designed to stick around in the body.
Suppliers usually ship this compound with purity above 98%, listing batch numbers, hazard pictograms, CAS number 214278-10-9, as well as recommended storage conditions—dry and tightly capped at 2-8°C. Most sheets highlight moisture sensitivity and warn against exposure to strong oxidizers. Molecular formula lands at C5H9F2N, molecular weight hovers near 125.13 g/mol. Labels may switch between synonyms, so it pays to match the CAS number with what your experiment actually calls for.
Research groups have tried a handful of routes, but most rely on starting with piperidine and hitting it with a fluorinating agent selective for the 4-position. DAST (diethylaminosulfur trifluoride) gets a lot of use, as do reagents like Deoxo-Fluor. A precursor—usually a 4-hydroxypiperidine or a 4-halopiperidine—meets the fluorinating agent under controlled temperature, and monitoring is a must to avoid over-fluorination or ring scission. Purification normally rolls out through distillation or flash column chromatography, finished with GC-MS or NMR to verify purity and structure.
The C-F bond here refuses to give in to most nucleophiles, but the nitrogen still can act as a nucleophile or a ligand under the right conditions. That lets chemists use 4,4-difluoropiperidine as a scaffold, typically modifying the nitrogen with alkyl, aryl, or acyl groups. Reductive amination, N-alkylation, as well as cross-coupling reactions show up often in published work. I’ve seen colleagues eager to introduce this backbone into molecules aimed at improved metabolic stability—the fluorines serve as bulky protectors and help resist enzymatic oxidation.
Chemical catalogs and patent filings aren’t always on the same page, but common aliases include 4,4-difluoropiperidine, 1-piperidine, 4,4-difluoro-, and the fun-to-read 4,4-Di-F-piperidine. Some manufacturers stick to the CAS number to sidestep confusion. A quick scan of product listings in Sigma-Aldrich or TCI will show both the IUPAC name and a handful of these variants, so double-checking structures or registry numbers goes a long way to avoid a mix-up—especially if you’re scaling from benchwork to pilot batch.
Handling 4,4-difluoropiperidine demands respect for its volatility and mild toxicity. Liquid gloves, goggles, and a fume hood become standard fare. Splashes or spills can irritate skin or lungs, and inhalation at higher concentrations brings on respiratory irritation. Standard operating procedures highlight rapid venting and containment of any spills, as well as safeguards against any buildup of vapors. Most data sheets mention moderate aquatic toxicity, so proper disposal routes through hazardous waste streams, rather than the drain, stay mandatory. For storage, tightly sealed amber vials inside secondary containment keep air and light from chewing up the product.
The real action shows up in the world of drug discovery. Medicinal chemists turn to 4,4-difluoropiperidine to craft molecules that need a boost in stability, or sometimes to block metabolic spots where enzymes usually strike. This difluoro twist has worked its way into candidate drugs across fields from psychiatric medicine to oncology. Outside human health, research groups have dabbled with it as a component in new materials, such as specialty polymers or as intermediates in agrochemicals. Analytical chemists sometimes use it to study how fluorinated motifs tweak hydrogen-bonding and acidity, feeding data back into broader molecular design strategies.
Academic labs and big pharma outfits both hunt for inventive ways to work with fluorinated piperidines. Structure-activity relationship studies have included 4,4-difluoropiperidine as a key variable, comparing its biological profiles to that of methyl, chloro, or plain hydrogen analogs. I’ve watched research groups tinker with automated synthesis robots to explore hundreds of analogs, and this building block grabs regular attention for providing both metabolic shields and new routes for chain extension. A batch of recent papers focuses on coupling reactions that swap out the relative inertness of C–F for something more interactive, nudging future modifications closer to reality.
Safety data remains in short supply, but animal models tell us a few key things. Acute exposure brings moderate irritation, with some liver enzyme changes at high oral doses. Metabolism studies point out that the difluorinated carbon resists rapid breakdown, which supports its use in medications but raises potential questions about accumulation. So far, testing at levels relevant for lab work points to a compound that’s no worse than many similar chemicals, provided proper care accompanies its use. Environmental fate hasn’t been fully charted, but like most fluorinated organics, this molecule shows persistence, so attention to waste management stays necessary.
Look ahead and there’s little doubt that interest in 4,4-difluoropiperidine will keep spreading. Movement toward fluorine-rich drug candidates shows no sign of slowing down, and the steady push for “smart” molecules—where subtle atomic changes tip the whole profile—fits right into the wheelhouse of this compound. New catalytic methods might open up easier access or new types of derivatization. Environmental regulators and industrial toxicologists will likely step up scrutiny as use increases. What started off as a curious exercise in fluorine placement has grown into a genuinely useful bit of chemistry, and there’s every reason to expect that tomorrow’s medicines, materials, and maybe even imaging agents will keep finding space for this tidy little ring and its pair of fluorine atoms.
Fluorine atoms change how a molecule behaves, both in the lab and out in the world. I’ve seen firsthand how these little tweaks—such as swapping in two fluorine atoms in just the right spots—can turn a basic building block into something far more interesting. 4,4-Difluoropiperidine starts out sounding like a mouthful, but its chemistry isn’t as mysterious as you might think.
At its core, 4,4-Difluoropiperidine is a piperidine derivative. Piperidine itself comes shaped like a six-sided ring, kind of like a stretched-out hexagon with one nitrogen and five carbons. In this version, those two fluorine atoms each latch on to the fourth carbon in the chain, sitting as neighbors on the same carbon. This setup gives the molecule some special properties, since fluorines are notoriously stubborn and make strong bonds. They send a ripple effect through the ring, often making the molecule less likely to break down in biological systems.
Take it from daily lab experience—placing fluorine where carbon once stood isn’t about just swapping parts. These changes crank up the molecule’s resistance to metabolic enzymes, so it sticks around longer inside living bodies. That plays a role in pharmaceuticals, where you want a drug that doesn’t disappear before it finishes its job. In other contexts, chemical durability helps ease concerns about stability for storage and transport.
Flip open any chemical database, and you’ll find the formula for 4,4-Difluoropiperidine as C5H9F2N. This compact set of atoms hints at its simplicity but also highlights that those two fluorines aren’t just thrown in randomly—they really do define its identity. It’s not a long or unwieldy molecule, which makes it handy in organic synthesis or as a stepping stone for bigger chemical projects.
In a practical sense, these details matter because formulas and structures are like addresses on a map. As someone who values accuracy in both the classroom and the lab, I know the difference between mistaking a methyl for a fluorine group can throw an entire experiment sideways. 4,4-Difluoropiperidine’s formula and setup give a clear picture for chemists looking for predictable results or consistent reactions.
Medicinal chemists love molecules like this because they show how small edits bring big outcomes. Drop in those fluorines, and suddenly the molecule dodges the body’s detox machinery and handles heat in reactions better than its unmodified piperidine cousin. Sometimes, it’s also about chasing intellectual curiosity; plenty of discoveries start with someone wondering, “What will happen if I do this …?”
Across my experience, a recurring challenge is keeping up with increasing regulatory demands for safer, cleaner, and more effective chemicals. Molecules engineered with tough bonds—like 4,4-Difluoropiperidine—help address some of these hurdles. While the overuse of persistent chemicals creates new environmental puzzles, strategically placed fluorine often means reduced need for heavy doses or risky additives.
Practical solutions come from a careful balance. Developing less-persistent alternatives or creating pathways for targeted breakdown under specific conditions makes sense for a world wanting clean chemistry. At the same time, picking simple, well-defined molecules—like this difluorinated ring—lets research keep moving without sacrificing precision.
4,4-Difluoropiperidine doesn't get much attention outside chemistry circles, but its impact reaches further than most realize. This molecule, with a couple of fluorine atoms on its piperidine ring, forms a core building block for a wide range of chemical pursuits. Whenever I hear about a new pharmaceutical breakthrough, there's a good chance that clever molecules like this are working in the background, shaping the whole research landscape.
Drug makers constantly look for new compounds to address tough diseases. The addition of fluorine atoms to simple molecular frameworks changes how a drug acts inside the body. Fluorine’s presence often boosts the stability of a medicine or improves how it’s absorbed. Medicinal chemists lean on 4,4-difluoropiperidine because it offers a way to introduce these properties into new drug candidates. For instance, companies exploring therapies for neurological disorders, cancer, or infectious diseases often turn to this molecule during early testing. Its structure may seem simple, but the benefits show up during rigorous lab tests. My years in academic labs always confirmed this: small tweaks in molecular design can lead to a world of difference.
Take a look at agricultural chemistry and you'll find a similar trend. Modern crop protection pulls from the same chemical toolbox as drug design. Researchers use specialized building blocks to create new pesticides or herbicides with specific effects on pests or weeds. 4,4-Difluoropiperidine works well in this space too, improving the action and environmental persistence of active ingredients. The key advantage is fine control. Farmers need solutions that target only what’s necessary and don’t linger in the soil or water. That takes smart chemistry during development, and 4,4-difluoropiperidine often becomes part of that equation.
Fine chemical manufacturers keep stocks of intermediates like 4,4-difluoropiperidine on hand for custom synthesis work. Whenever a research lab or startup ventures into new territory, these companies produce tailored amounts—sometimes a few grams, sometimes enough for tons of product. This flexibility supports the entire innovation pipeline. From smaller research projects to the pilot-scale production that comes right before full market release, these chemicals support real progress. Companies turn to trusted suppliers not just for material itself, but for technical know-how about handling, purity, and safe-scale-up. Safe practice and quality control prevent unwanted surprises in sensitive processes.
Years ago, environmental conversations around chemicals barely reached the mainstream. Now, expectations change quickly, especially in how labs and factories use fluorinated building blocks. Safer synthesis routes, less waste, and responsible sourcing influence how chemists select materials. Plenty of teams now seek ways to recover or recycle valuable intermediates, including difluorinated piperidines. Solutions involve designing cleaner reactions or turning to biocatalysis—letting engineered enzymes do some of the hard work, often with fewer toxic byproducts. Everyone in the chemical supply chain sees the benefit in less pollution and safer working conditions. Each step in production, from sourcing to disposal, can reflect these values.
My own experience reminds me that progress in chemical science isn’t just about headline discoveries—it builds from the quiet reliability of essential building blocks. 4,4-Difluoropiperidine powers advances across research areas, weaving its way into real products and everyday impact. Keeping a close eye on materials like this, from sourcing to safe use, makes sure the benefits outweigh the risks as technology evolves.
4,4-Difluoropiperidine looks like any other lab chemical on paper, but its properties and potential hazards deserve more than a quick glance at a safety sheet. From day one in any lab, I've seen how an ordinary compound can turn a routine task into something much riskier without careful handling. This particular chemical gives off fumes, so a whiff in a closed space might do more than just wrinkle your nose—it can actually threaten your respiratory health. Accidentally splashing a little on your skin or in your eyes creates another set of real problems. There’s a reason so many injuries come from "simple mistakes" in the lab: the basics get ignored.
Putting on a lab coat isn’t just for show. For 4,4-Difluoropiperidine, it takes a full kit: nitrile gloves, safety goggles, a fitted coat, and, often, a face shield. Splash-proof safety glasses matter because regular glasses don’t block side splatters. If a chemical slip happens, skin and eyes should stay untouched. Always check gloves for holes or discoloration before diving in—this stuff soaks through unnoticed tears faster than you’d expect. I remember a colleague who forgot about a tiny tear and learned quickly that irritation doesn’t care if you’re running on a deadline.
Ventilation stands as a simple but often overlooked step. Fume hoods get neglected by people rushing around or trying to save space, and that’s how exposure creeps in. Whenever handling 4,4-Difluoropiperidine, running all work through a certified and functioning fume hood keeps the workspace livable—nobody wants to chase mystery headaches caused by vapor inhalation. Fume hoods aren’t luxury items; they’re barriers between you and some nasty outcomes. If the hood’s alarm goes off or the airflow drops, don’t play the odds. Move away and get it checked out every time.
Every so often, someone treats spills like a quick mop-up job. With compounds like this, absorb spills using chemical-resistant pads, not whatever cloth is handy. Discard any cleaning material as hazardous waste—don’t toss it into regular trash. Storing the chemical also means paying attention: keep it away from moisture, open flames, and direct sunlight. Shelves for storage should have lip guards, and labels need to stay clear and up to date.
Over time, training blends into the background. But without it, even experienced chemists slip up. New staff working with 4,4-Difluoropiperidine have to know emergency procedures, not just hazard symbols. Labs should carry clear instructions near workstations, not just in a hefty file nobody opens. Emergency showers and eyewash stations belong close and inspection happens every week, not just on paper for compliance. If a spill or exposure occurs, quick reactions save more than a report—they protect eyesight and lungs.
It’s easy to overlook the boring basics because everyone wants a faster workflow. Yet, the health cost isn’t a fair trade-off. I’ve seen cuts from shortcuts end in more regret and paperwork than any saved time was worth. Safe handling of 4,4-Difluoropiperidine means routine, caution, and a little pride in doing each step right. Working as a team and having honest conversations about close calls or unsafe habits helps everyone make better choices.
Preventing problems means constant reminders, not just annual safety refreshers. Brief safety meetings before big projects keep everyone sharp. Posting clear signage, making spill kits and first aid gear visible, and holding small group drills help the lessons stick. Sharing stories of near-misses creates real awareness—every lab tech I’ve worked with can remember at least one. Safety grows into a habit when nobody lets the rules slide, even on a busy day.
4,4-Difluoropiperidine usually shows up on lab benches for a reason—it brings unique properties chemists look for when working on drug molecules. But a simple misstep in storage or transport can turn a useful compound into a real safety concern. I've spent time around chemical storerooms and transport docks, and I've seen how shortcuts can backfire. Leaky bottles, poorly labeled drums, and improper containers can all lead to hazardous spills or unwanted reactions. Every step from shelf to shipment throws out different risks. Here’s where science meets daily common sense.
You won’t see experienced chemists keeping 4,4-Difluoropiperidine next to their snacks in the fridge. This compound shows sensitivity to moisture and can turn nasty if exposed to the wrong environment. Glass or high-quality plastic containers with tight-fitting, chemical-resistant caps hold up well. Even on a small scale, you don’t cut corners with labels. A clear, legible label with the name, date, and hazard info stops confusion before it starts.
Well-ventilated storage cabinets, away from direct sunlight and sources of heat, play their part. No one wants toxic fumes or accidental heating. Chemical-grade refrigerators or cool, dry storage rooms usually do the trick, keeping the bottle below room temperature, around 2–8°C if data sheets back it up. Every storage setup should come with a spill kit nearby. Absorbent pads, neutralizers, and gloves belong within easy reach for quick clean-up if something tips over.
Once you leave the lab or storeroom, the real challenges begin. I've watched shipments change hands at loading docks and seen how a missed detail can lead to leaks or breakage. Certified chemical carriers who understand hazardous materials make all the difference. They use cushioned, leak-proof containers and tamper-evident seals.
Proper documentation travels along with every package. I once saw a shipment delayed for days because the paperwork didn’t match the latest regulations. For international shipping, understanding both departure and arrival country requirements avoids endless headaches— paperwork, safety data sheets, and packaging all need to line up. Adding obvious hazard labels helps ensure even non-specialists take extra care during handling.
The only way to avoid chemical accidents is through a mix of training, resources, and attention. I’ve seen staff briefed before handling chemicals—those short run-throughs help everyone avoid the classic mishaps. Digital inventory helps track location and age of every bottle or drum, cutting down on expired stock or missing goods.
Some companies install chemical sensors or automatic ventilation in storage rooms. These setups aren’t fancy extras; they help catch problems early—fumes, temperature spikes, or accidental leaks—before anyone gets hurt or sick. Regular, scheduled checks by experienced staff can spot rusted locks, cracked containers, or building humidity. Even the best system works better with human eyes and hands on the job.
4,4-Difluoropiperidine isn't something to take lightly. Simple respect—good labeling, sturdy packaging, the right compartments, and trained hands—keeps the workflow safe and smooth, whether in a startup lab or a crowded shipping hub.
I’ve worked with a range of chemicals, from off-the-shelf solvents to custom-ordered reagents, and anyone who’s spent time in a research lab knows how a small detail on a label can grow into a large headache if it’s not right. Take 4,4-Difluoropiperidine. You’ll spot it in synthesis projects, drug discovery, and sometimes in pilot pharmaceutical manufacturing. Here, nobody wants surprises in the material. The difference between 95% and 99% purity can be the difference between a clean product and a clogged-up reaction. Some chemists start with technical grade, figuring they’ll purify things later. Others insist on analytical grade from the outset. The stakes go up as the chemical moves further from benchtop experiments and closer to something destined for human use.
Buying straight from big suppliers often lets you pick a standard purity, maybe 97%. Some customers go straight to the sales team and ask for a higher grade. I’ve seen larger groups order in bulk, pooling resources to score higher purity at a better rate. Smaller research outfits get fewer options and bigger costs per gram. Some specialty suppliers even offer to prepare a custom grade. It gets expensive, and the timeline stretches, but sometimes the project can’t go forward any other way.
A lot of people outside the lab think about bigger bottles meaning more savings. Someone who goes through a kilo a month doesn’t worry much about leftovers, but most research projects crawl along, using a few grams here and there. I’ve seen full bottles of a rare compound expire on a shelf, all because the smallest package was still ten times what the team needed. Besides wasting money, storing unneeded chemicals takes up space and raises questions from the safety committee. That’s not just a paperwork issue — mishandling reactive or volatile compounds can end up in an emergency call.
Labs that focus on small-scale synthesis often look for 5- or 10-gram vials. Some suppliers have no patience for this: they’ll only sell 100 grams or more, figuring the paperwork isn’t worth their time. This leaves smaller labs to either overpay or club together to split the order, then argue about freezer space. Packaging sizes that match actual research needs go a long way toward keeping things running smoothly. Nobody wants to pay for a large drum that becomes hazardous waste two years later.
Access to different grades and container sizes turns on a few levers. It always makes a difference to ask directly. Suppliers often keep standard sizes on the website but will break bulk on request or find smaller vials behind the scenes. Pricing isn’t always transparent, especially on custom grades. Researchers with unusual needs sometimes work with a distributor to get a batch split or persuade a supplier to add a new option if there’s enough interest. There’s real value in building relationships beyond the online shopping cart.
If more chemical vendors remembered the shoestring budgets and modest storage spaces that most labs deal with, there wouldn’t be so many full containers left gathering dust. Meeting the customer halfway, with flexible sizing and honest conversations about purity, clears a lot of roadblocks — and more projects can move forward without delays from sourcing hiccups.

| Names | |
| Preferred IUPAC name | 4,4-Difluoroazacyclohexane |
| Other names |
4,4-Difluorohexahydropyridine 1,1-Difluoropiperidine |
| Pronunciation | /ˈfɔːr fəˌlʊɔːr paɪˈpɛr.ɪˌdiːn/ |
| Identifiers | |
| CAS Number | 2869-15-6 |
| 3D model (JSmol) | `3DJN` |
| Beilstein Reference | 1364803 |
| ChEBI | CHEBI:139474 |
| ChEMBL | CHEMBL186152 |
| ChemSpider | 142080 |
| DrugBank | DB14666 |
| ECHA InfoCard | 03b60b2d-187e-4c77-84ee-8e9b7a5792b7 |
| EC Number | 695-912-8 |
| Gmelin Reference | 120247 |
| KEGG | C19438 |
| MeSH | D08.811.277.040.500.500 |
| PubChem CID | 10411012 |
| RTECS number | TM3150400 |
| UNII | H2K4C112CS |
| UN number | UN3272 |
| CompTox Dashboard (EPA) | DTXSID70869595 |
| Properties | |
| Chemical formula | C5H10F2N |
| Molar mass | 123.14 g/mol |
| Appearance | Colorless liquid |
| Odor | Amine-like |
| Density | 1.16 g/mL at 25 °C(lit.) |
| Solubility in water | Slightly soluble |
| log P | 0.33 |
| Vapor pressure | 1.87 x 10² mmHg at 25 °C |
| Acidity (pKa) | 11.2 |
| Basicity (pKb) | 2.65 |
| Magnetic susceptibility (χ) | -68.7·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.396 |
| Dipole moment | 2.54 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 329.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -604.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3333.0 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05 |
| Signal word | Danger |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P210, P261, P280, P305+P351+P338, P337+P313, P403+P233 |
| NFPA 704 (fire diamond) | Health: 2, Flammability: 2, Instability: 0, Special: -- |
| Flash point | 57 °C |
| Autoignition temperature | Autoignition temperature: 515 °C |
| LD50 (median dose) | LD50 (median dose): Oral rat >2000 mg/kg |
| NIOSH | NA0272000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | NIOSH REL 5 ppm (19 mg/m3) 8-hr TWA |
| Related compounds | |
| Related compounds |
Piperidine 4-Fluoropiperidine 3,4-Difluoropiperidine Perfluoropiperidine Piperazine N-Methylpiperidine |