Discovery and use of morpholinium derivatives began at a time when synthetic chemistry set its sights on new frontiers for pharmaceuticals and agriculture. Early literature pointed to the interest in chloroalkylated amines because of their reactivity and their capacity to latch onto biological targets with precision. Morpholine, a basic building block in organic synthesis, found itself modified by various groups through the decades. The appearance of 4-(2-chloroethyl)morpholinium chloride in scientific reports signals a shift from purely experimental use to one rooted in practical applications. I dug through chemistry archives and patent filings; most trails lead back to European and North American labs in the late 1960s. Since then, researchers have experimented with formulations, looking for better reactivity and safety profiles. That pursuit still shapes today's production methods, where purity and stability matter just as much as cost and yield.
4-(2-Chloroethyl)morpholinium chloride carries the weight of an industrial chemical with a hand in pharmaceuticals, polymer synthesis, and specialty reactions. This compound does not make headlines, but its unique structure—a morpholine ring with an appended 2-chloroethyl group, paired with a chloride ion—serves as both a reactive intermediate and a functional additive. Research chemists have tapped into its properties to drive alkylation reactions, where the chloroethyl chain enables nucleophilic substitution. In my experience researching similar quaternary ammonium salts, the value lies in their dual nature; their ionic character aids solubility in water, and their organic skeleton permits modification for tailored outcomes.
Pure 4-(2-chloroethyl)morpholinium chloride appears as a white to off-white crystalline powder, stable under normal laboratory conditions, with a faint amine odor. The salt dissolves well in water and polar solvents, which streamlines handling during preparation and application. Its melting point generally falls between 200°C and 220°C, depending on trace impurities. Such thermal stability has practical value in multi-step syntheses, resisting decomposition until activated by stronger reagents. The molecule’s basicity, imparted by the morpholinium ring, often gets tested in titrations, and I’ve found it resists hydrolysis better than many open-chain analogs. Handling this compound, you appreciate how the chloride counterion not only neutralizes charge but also affects solubility patterns in various solvent systems.
Manufacturers formulate technical grades with typical purity levels above 98%. Labels spell out molecular formula—C6H13Cl2NO—along with batch number, storage instructions, and hazard codes consistent with global guidelines. Containers usually bear recommendations for storage below 25°C, away from oxidizers, and out of direct sunlight. On-site, I’ve seen bottles packed with desiccants to forestall clumping. Operators pay attention to the detailed safety data, which should be clear, legible, and compliant with regulations set by agencies like OSHA, REACH, or GHS. Lot-to-lot consistency gets tracked by NMR and HPLC, with every data sheet providing spectral characteristics, trace metal content, and detailed instructions for safe disposal.
The classic route involves the alkylation of morpholine using 1,2-dichloroethane, often under reflux in an aqueous or alcoholic medium. Stoichiometric quantities ensure minimal leftover starting material and avoid secondary products. Researchers introduce a base, typically sodium carbonate, to neutralize liberated HCl. In my time working on quaternary salt syntheses, careful control of reaction time and temperature makes or breaks yield—run too hot or long, and you end up chasing purification headaches. Isolation happens through solvent evaporation, followed by crystallization from ethanol or acetone. Labs scale the process with tweaks tailored to purity and regulatory requirements. The bake-out step, removing water, guarantees shelf stability.
The core reactive site, a 2-chloroethyl group, makes this compound a popular alkylating agent for nucleophilic substrates—amines, thiols, even some metal complexes. Once introduced into a reaction, the chloride atom leaves, enabling covalent bond formation. In custom syntheses, research groups take the morpholinium framework and replace the ethyl or chloride to build libraries of similar quaternary salts, tuning properties like water solubility or bioactivity. In the pharmaceutical sector, modifications can optimize membrane permeability or reduce toxicity. Experience shows the balancing act between reactivity and selectivity poses serious challenges; one step too far and byproducts begin dominating the reaction mass. Still, with diligent kinetic studies, chemists use its reactivity to install functional groups onto everything from simple building blocks to complex, chiral molecules.
In chemical supply catalogs and journal articles, this compound appears under several monikers. Morpholinium, 4-(2-chloroethyl)-, chloride is a frequent alternative, and various systematic names circulate in regulatory filings. Trade names change by region and supplier; some call it Morpholine, N-(2-chloroethyl)-, hydrochloride salt, while others stick with direct chemical descriptions. This variety complicates database searches; mislabeling risks ordering errors and lab confusion, as I’ve experienced firsthand. Careful cross-verification with CAS numbers and molecular weight data keeps operations on track and avoids costly mistakes, especially during scale-up or compliance checks.
Handling 4-(2-chloroethyl)morpholinium chloride demands vigilance, as the 2-chloroethyl group triggers health and environmental concerns. Skin contact leads to irritation and possible sensitization, so gloves and face shields stay mandatory. I’ve learned to respect the inhalation hazard—fine dust carries respiratory risks and can trigger asthma-like symptoms. Labs rely on robust fumehoods, spill response kits, and training on emergency procedures. Material Safety Data Sheets require frequent updates to reflect evolving toxicology data. Disposal channels route waste through licensed handlers, avoiding direct release into waste streams. Regulatory frameworks shoulder most of the burden here; regular audits and record-keeping reinforce a culture focused on worker and community safety. National and international guidelines dictate recordkeeping, storage duration, and allowable concentrations in ambient environments.
Pharmaceutical synthesis uses 4-(2-chloroethyl)morpholinium chloride for preparing anti-cancer agents, especially in pathways assembling nitrogen mustards. The compound finds a place in producing specialty resins and as a template in supramolecular chemistry. I’ve talked to process chemists from the agrochemical sector; their projects rely on its reactivity for preparing intermediates in herbicide or growth regulator formulations. Outside core industry, academic groups explore the salt in polymer modification and as a coupling agent for experimental biocatalysts. End users span multinational pharma firms, mid-sized contract manufacturers, and university research labs, each demanding slightly different performance parameters.
Current R&D takes two tracks: optimizing synthesis for greener chemistry and finding new biological applications. Automation in process chemistry brings real gains to yield and safety in pilot plants. I’ve followed projects swapping conventional solvents for low-toxicity, biodegradable options, where both efficiency and regulatory compliance improve. On the biological side, collaborations between chemists and pharmacologists test morpholinium salts as prodrugs, with attention focused on cancer, neurodegenerative disease, and rare infections. Big data analytics now shape decisions in early discovery, leveraging predictive models on reactivity and toxicity. Companies file dozens of new patents every year, signaling confidence in untapped uses—especially where competitive advantage aligns with environmental stewardship.
Toxicological screening labels the compound as acutely toxic by ingestion, with known effects on liver and kidney tissue at higher doses. Animal models reveal dose-dependent impacts, including neurological symptoms and, at extreme levels, organ failure. In my view, the most thorough studies come from collaborations between academic toxicologists and industrial safety teams. Chronic exposure studies show potential carcinogenic effects and cytotoxicity against fast-dividing cells, which steers its use toward controlled laboratory settings and away from consumer products. Wastewater monitoring in production sites finds the molecule persists if untreated, raising red flags for aquatic life. Each fresh set of toxicology data sharpens the rules factories follow to keep both workers and the environment out of harm’s way.
Growth in specialty pharmaceuticals and the need for new molecular scaffolds both fuel demand for morpholinium derivatives. Green chemistry will force changes in how these compounds are made and handled; already, process engineers design recycling protocols for solvents and aim to cut water usage. I see targeted modifications on the horizon, aiming to tune activity profiles and minimize unwanted biological side effects. Regulatory scrutiny tightens year by year, so future research must address long-term fate in ecosystems. Digital tools, from computational modeling to automated synthesis, give labs resources for testing safety and reactivity in silico before a single flask gets filled. Expanding uses in medicine, plastics, coatings, and energy storage keep this field alive, with continuous efforts to balance innovation, human health, and environmental responsibility.
Some chemicals get plenty of attention, showing up in headlines because of their role in medicine, crops, or the latest science discovery. Others, like 4-(2-Chloroethyl)Morpholinium Chloride, stay less visible, but that doesn’t mean they carry less weight. This compound pops up in a handful of serious jobs—tasks that often don’t get much spotlight, but absolutely make a difference behind lab doors and inside hospital walls.
Much of what you see about cancer drugs focuses on the “blockbusters”—those big-name medications you hear about during fundraising seasons or clinical trial updates. 4-(2-Chloroethyl)Morpholinium Chloride works differently. As part of the morpholinium group of chemicals, it’s drawn interest for its “alkylating” potential in chemotherapy. In my time spent shadowing pharmacists in oncology clinics, I learned how alkylating agents attack cancer cells by scrambling their DNA. The cells stop dividing, and the cancer slows or even shrinks. Scientists have looked at this particular compound and its cousins for those very abilities. It doesn’t often make it as a first-choice medication, but it’s valuable as part of the ongoing chemical investigations for new treatments.
Pharmaceutical companies and university research groups constantly try new structures to fight disease. Sitting in the library, flipping through journals, I noticed studies from Eastern European labs experimenting with this chloride for its anti-tumor and antifungal activities. Results read mixed at times, but the drive to test new chemicals—no matter how obscure—pushes medicine forward.
Not every new agent finds its place on pharmacy shelves, and side effects or stability issues keep some ideas off the market. Screening compounds like 4-(2-Chloroethyl)Morpholinium Chloride helps scientists map what works against cancer or infections, and what doesn’t.
Some companies lean on chemicals like this for analysis and synthesis, not direct therapy. Talking to chemists over coffee, I’ve picked up that 4-(2-Chloroethyl)Morpholinium Chloride sometimes acts as an intermediate step to build more complex molecules. Laboratories shape it into dyes, specialty coatings, and materials you need for other experiments. The compound’s unique structure plays a part by linking to other chemicals in creative ways.
Most people never handle this type of chemical, and that’s a good thing. Inhalation, direct skin contact, or accidents can bring real danger. The chloride’s chemical activity—what makes it useful—also brings toxicity. Lab workers dress up in gloves, goggles, and coats for a reason. They store every bottle away from moisture and strong acids, and they keep records about spills or mishaps. Safety data reminds everyone: Approach with care.
As researchers grind forward, testing every promising compound, the need for caution grows. Strong oversight from regulatory agencies makes a key difference. Reports from the World Health Organization and national bodies often guide research to keep both people and the environment safe. At the same time, collaboration between academic centers, pharmaceutical producers, and chemical safety experts keeps the field moving ahead responsibly.
Nobody’s likely to see 4-(2-Chloroethyl)Morpholinium Chloride in their home medicine cabinet. Yet, the quiet compounds—those tucked away on cold shelves—often stand at the root of progress in cancer care and chemical research.
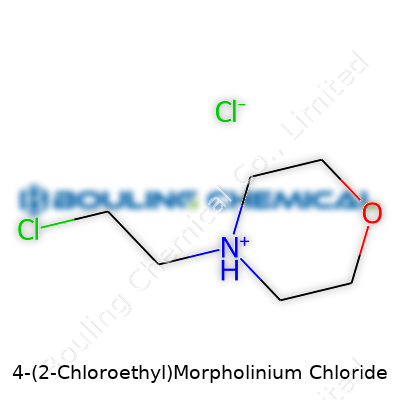
The name 4-(2-Chloroethyl)morpholinium chloride sounds dense, but this compound contains building blocks we see across a lot of chemical spaces. The backbone, morpholine, carries significance in pharmaceuticals, crop protection, and material science. What stirs interest here is the addition of a 2-chloroethyl substituent at the fourth position and the formation of a salt with chloride.
Looking closer, the morpholine ring gives the main structure. It brings a six-membered ring with four carbon atoms, one oxygen, and one nitrogen. Chemists know this ring for its stability and for helping drugs and dyes slip through biological membranes. Adding a 2-chloroethyl group to the nitrogen kicks in extra reactivity. In daily lab work, this changes how scientists approach synthesis and what kind of biological action might show up in studies.
The actual molecule looks like this: the morpholine ring, shaped almost like a flat chair, with the nitrogen atom sticking out, connects to a two-carbon chain. On the end of that chain, a chlorine atom sits. This turns the molecule into a quaternary ammonium salt once it meets chloride, balancing the charge. A chemist sees this as a cation, often written as C6H12NOCl+ with Cl- as the counterion.
Visualizing this helps. Picture the morpholine ring as the core. The nitrogen at the fourth position bonds to a -CH2CH2Cl group. At the same time, the extra positive charge from quaternization sits on the nitrogen, which shifts some electron density and increases the water solubility.
Experience in drug discovery shows that a small tweak—like putting a chloroethyl group on the morpholine ring—totally resets how a molecule behaves. Suddenly, the molecule dissolves more easily. It enters cells in new ways. That positive charge doesn't just help it mix with water; it often means the molecule finds targets in the body differently. I've watched teams debate for days about moving a substituent around a ring because a slight change could mean a dead-end or a breakthrough in toxicity or activity.
Adding the chloride makes the material shelf-stable and easy to handle. Salts like this tend to hold steady at room temperature and in transport—something anyone working with reagents or active ingredients quickly learns to appreciate. Missing out on salt form selection during development can cost months when things go wrong in storage or formulation.
The presence of the chloroethyl group points toward alkylating properties. Chemically, this can lead to strong reactivity. Substances like these sometimes fall under scrutiny since similar structures can damage DNA. In the lab, gloves and fume hoods become non-negotiable. Companies need to investigate all toxicology data and make sure procedures limit any spills or skin contact.
Good process chemistry looks out for ways to trap any excess reagents and to neutralize waste streams. Publishing full safety data and sharing handling tips among scientists limits harm, especially as demand grows for useful building blocks in pharmaceuticals and specialty chemicals.
Knowing this structure ties into daily decisions about research and industry. Understanding it means less trial and error. My own experience tells me the best results happen when teams approach molecules with both practical and thoughtful eyes, respecting the fine line between the helpful and the hazardous. The way 4-(2-Chloroethyl)morpholinium chloride fits together shapes what it can do—and what those handling it need to watch.
Many chemical names spark concern, especially if you don’t work in a lab or chemical plant. 4-(2-Chloroethyl)morpholinium chloride sounds intimidating, and its structure suggests things worth paying attention to. My experience working with both research and safety compliance taught me that the devil really is in the details: structure, exposure, and use all count.
4-(2-Chloroethyl)morpholinium chloride carries a chlorinated ethyl group. Based on structure alone, you might spot similarities to known harmful agents like certain alkylating drugs or even nerve agents. That shouldn’t startle anyone outright, yet it does mean extra care is called for. Skin contact, inhalation, or accidental ingestion all represent realistic exposure routes for people handling this stuff.
Handling chemicals like this outside a lab isn’t common, but inside research, pharmaceutical, or chemical manufacturing workspaces, you’ll find people directly interacting with compounds in this family. The main question: can it move through the body and harm vital organs? Data points to the risk of tissue irritation, and at high enough doses, long-term effects can’t be ruled out. The United States National Library of Medicine assigns a hazard alert to similar structures, noting corrosive effects on eyes, skin, and the respiratory tract. Experience with morpholinium compounds supports that finding.
Toxicology research focuses on what happens when cells or tissues encounter these molecules. Chloroethyl groups react with DNA. That’s not just a technical detail. Enough reactivity like this can lead to mutations, cell death, or worse, slow-developing outcomes like cancer. Nobody in safety wants to be caught unprepared, so labs often use hoods, gloves, and other gear when measuring or mixing even small amounts.
Publicly available literature offers little on specific fatalities caused by 4-(2-chloroethyl)morpholinium chloride, but there are case studies and chemical analogues linked to severe outcomes. Regulatory guidance places these compounds on lists requiring hazard communication and proper training. In Europe, for instance, similar chlorinated organic compounds fall under REACH controls.
Trust in engineering controls goes a long way. I’ve seen workspaces where one careless rush led to a costly, days-long cleanup because someone underestimated a chlorinated morpholinium salt. In those cases, splash goggles and gloves never seemed excessive. Beyond that: routine air monitoring and spill plans support the idea that prevention beats treatment.
Disposal matters. Sewer and landfill won’t cut it, both due to possible hazardous breakdown products and environmental persistence. Professional hazardous waste disposal companies exist for a reason—costs go up, but so does long-term safety.
Transparency and access to accurate hazard data remain issues. It shouldn’t take a background in chemistry to understand the dangers. If companies introduce new chemicals into the marketplace, they must test and share results plainly. Some folks call for stricter consumer labeling and easier access to Material Safety Data Sheets. These approaches turn knowledge into safer behavior, whether a compound is on a research bench or in an industrial drum.
Experience in chemical safety tells me: if a compound looks risky, treat it that way. Vigilance, training, and clear rules cut injuries and illness. 4-(2-Chloroethyl)morpholinium chloride doesn’t get a free pass; it gets respect, caution, and real education before use.
Handling chemicals like 4-(2-Chloroethyl)Morpholinium Chloride teaches you quickly that careless storage leads to bigger troubles than missing inventory. Years working around hazardous substances have shaped the way I see even basic lab routines—nothing about this compound invites shortcuts. With a history in organic synthesis and plenty of run-ins with compounds that sound just as technical, experience tells me this one deserves full respect from the start.
Chemicals containing a chloroethyl group often carry a reputation for being both reactive and sometimes carcinogenic. For this one, dry, well-ventilated shelves win out over just tossing it in any cabinet. Direct sunlight can prompt decomposition, and a warm storeroom may nudge the compound into breaking down faster than expected. Temperatures stay at room level, or slightly cooler if possible—no swinging between hot and cold, since condensation inside a container leads to unwanted reactions.
Humidity brings its own set of risks. Moist air sneaks in, especially with screw-tops that don’t quite seal. Corrosive vapors from other bottles will speed up unwanted changes, so storing it away from acids or strong bases works best. If you ever worked with hydrochloric acid nearby, the lesson is clear—cross-contamination ruins more than product quality; it can mess with basic safety.
A clear label with a hazard symbol means nobody confuses it for a less reactive salt. In the same way that in any kitchen you wouldn’t pour drain cleaner in a water pitcher, mistakes with labeling here have far-reaching consequences. Containers made of high-quality plastic or safety glass play a major role, because chemical-grade glass won’t pit or cloud. Plastic should stand up to strong alkylating agents; skip anything recycled or questionable here.
Original packaging serves as a sort of shield—custom fit, tighter caps, and often extra layers to soak up moisture in transit. That packing paper or foam isn’t just for shipping; sometimes it acts as a last barrier against leaks.
Direct skin contact with compounds like this feels risky, having seen enough minor accidents escalate. Nitrile gloves, safety goggles, and wearing a lab coat don’t just show caution—they simply prevent burns, allergic reactions, and worse. Think back to anyone who scoffed at facial protection and got a whiff of irritating fumes up close. Fume hoods protect even better, keeping vapors away and dropping exposure from accidental spills.
Work spaces decked out with shower heads and eye-wash stations seem excessive, until someone splashes something caustic. Planning ahead never gets old. Spills cleaned immediately with absorbent material avoid anything soaking through surfaces or spreading to other chemicals.
Pouring leftovers down the drain sends harmful agents straight to the local water supply. That crossed my mind once, but training and a couple of hard lessons steer disposal decisions now. Giving waste to professional hazardous materials teams means better peace of mind; they come equipped for proper neutralization and aren’t guessing what’s in the beaker.
Guidelines from groups like OSHA and the EPA keep people from improvising. That’s not bureaucracy getting in the way—that’s hard-won advice from years of accidents and close calls in chemical storage rooms across the world.
Familiar routines become second nature, but that’s exactly where slip-ups wait. When everything about your day starts with checking lids, watching the climate, or confirming labels, you earn quieter evenings—no sirens or emergency calls. Safety with chemicals like 4-(2-Chloroethyl)Morpholinium Chloride falls right in line with good habits, an understanding of risk, and the willingness to spend a minute longer double-checking.
4-(2-Chloroethyl)morpholinium chloride shows up in organic chemistry labs and some pharmaceutical research. With its capacity to alkylate, this compound finds utility in specific synthesis workflows that require precise handling, safety protocols, and legitimate purposes. It’s not something that high school chemistry classes will keep on a shelf, and knowing why brings a layer of respect for its potential risks.
The biggest hurdle in acquiring this compound comes from strict regulatory controls. Chemical suppliers, including those operating globally, need to verify buyers and their intent. Vendors often ask for institutional details, end-use declarations, and may refuse individual retail orders completely. To avoid headaches and legal risks, sourcing through a recognized institution—like a university lab or registered corporation—opens the door to a legitimate transaction. No reputable supplier will process orders without documentation, so pretending to represent a laboratory isn’t just easy to spot, it can get you into real trouble.
Many suppliers with a chemical catalog big enough to carry 4-(2-Chloroethyl)morpholinium chloride work exclusively with researchers who’ve built a relationship with them. Companies such as Sigma-Aldrich, Alfa Aesar, and TCI Chemistry have a public presence and clearly outline how to order, where they export, and what paperwork gets the ball rolling. Experience tells me that even for approved researchers, the due diligence at the purchasing stage feels like applying for a grant—intense and detailed. If you’re part of a research group, be prepared to share institutional credentials and project summaries.
The grey market surrounds specialty chemicals just like it does anything valuable or controlled. Buying from resellers or obscure online outlets means stepping into legal and safety quicksand. Without verification or traceability, you could end up with impure—or entirely different—material, or become the focus of a regulatory investigation. That path isn’t worth saving a few dollars or shortcuts.
Safety is another issue. Even legitimate supply raises the need for skilled navigation. The compound’s alkylating behavior, combined with its toxicity profile, creates hazards for your team and the wider community. Every organization I’ve worked with had a ChemTracker-style inventory, locked chemical rooms, and annual audits. Staff who mishandle toxic intermediates can expose themselves, and waste disposal rules bring their own mountain of paperwork. Training and documentation protect both people and budgets.
Some researchers look at in-house synthesis. This choice throws additional risks and lab requirements into the mix. Precursor controls, byproduct management, and waste stream tracking become non-negotiable. Experienced synthetic chemists see this as a last resort because of the burden of purity verification and the mountain of regulations for making anything on controlled substance lists.
Inside research teams, it’s not just about possessing what you need. The question focuses on proper stewardship and community safety. If in doubt, talk to your institutional chemical safety officer before placing any orders. An open, documented process gets the right suppliers, protects the research, and shields people from legal or health problems down the line.
| Names | |
| Preferred IUPAC name | 4-(2-chloroethan-1-yl)morpholin-4-ium chloride |
| Other names |
2-Chloroethylmorpholinium chloride Morpholine, 4-(2-chloroethyl)-, chloride 4-(2-Chloroethyl)morpholine hydrochloride |
| Pronunciation | /ˈfɔːr tuː ˈklɔːroʊˌɛθɪl mɔːrˈfɒlɪniəm ˈklɔːraɪd/ |
| Identifiers | |
| CAS Number | 3647-69-6 |
| 3D model (JSmol) | `CCC1NCCO1.Cl` |
| Beilstein Reference | 1721045 |
| ChEBI | CHEBI:65310 |
| ChEMBL | CHEMBL187984 |
| ChemSpider | 110057 |
| DrugBank | DB13131 |
| ECHA InfoCard | ECHA InfoCard: 100.039.860 |
| EC Number | 2536-99-4 |
| Gmelin Reference | 82164 |
| KEGG | C19229 |
| MeSH | D015703 |
| PubChem CID | 13061 |
| RTECS number | TD0875000 |
| UNII | 1V09E1J9JZ |
| UN number | 3276 |
| Properties | |
| Chemical formula | C6H13Cl2NO |
| Molar mass | 197.08 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.22 g/cm3 |
| Solubility in water | Soluble in water |
| log P | -2.2 |
| Acidity (pKa) | 7.4 |
| Basicity (pKb) | –0.5 |
| Magnetic susceptibility (χ) | -59.4×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.528 |
| Dipole moment | 6.53 D |
| Hazards | |
| Main hazards | Toxic if swallowed, causes skin and serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS06,GHS05 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P332+P313, P337+P313, P362+P364 |
| NFPA 704 (fire diamond) | 3-2-2 |
| Flash point | > 251.4 °C |
| Lethal dose or concentration | LD50 (oral, rat): 89 mg/kg |
| LD50 (median dose) | LD50 (median dose): 80 mg/kg (intravenous, mouse) |
| NIOSH | BO1460000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 4-(2-Chloroethyl)Morpholinium Chloride: "not established |
| REL (Recommended) | 0.05 ppm |
| Related compounds | |
| Related compounds |
2-Chloroethylmorpholine Morpholine Chloroethyl chloride N-(2-Chloroethyl)morpholine 4-(2-Bromoethyl)morpholinium bromide |