Chemists first started working with derivatives of morpholine in the early twentieth century. The goal focused on finding reliable intermediates for pharmaceuticals and polymers. Through decades of exploration, 4-(1-Oxo-2-Propenyl)-Morpholine emerged from targeted efforts aimed at crafting amide-functionalized morpholine compounds. While laboratory notes rarely mention the names of the bench chemists or the long hours refinement required, the efforts resulted in a molecule that attracted researchers from different sectors. It is fascinating that this compound gradually moved from dusty academic publications into pilot scale, showing how the chemical industry draws from persistent tinkering and a willingness to try out modifications season after season.
4-(1-Oxo-2-Propenyl)-Morpholine represents a molecule that bridges practical needs in organic synthesis with design ambitions in material science. Its backbone includes a morpholine ring—a six-membered structure with nitrogen and oxygen—linked to an acryloyl group through a robust amide bond. The resulting compound looks simple on paper. In labs, it shows formidable versatility in various reaction settings. This singular structure attracts both synthetic chemists and application developers, especially those searching for stability without sacrificing reactivity.
Solid at room temperature, this compound’s pale yellow crystals dissolve well in polar organic solvents like DMSO and DMF. The odor can best be described as faintly amine-like. It melts between 40 and 44°C—easy to handle but cautious precision matters near its melting point. The structure ensures strong resonance stabilization across the amide bond, and the acryloyl moiety presents a reactive site for polymerization and Michael-type reactions. The logP value places it in a moderately hydrophilic zone, which influences its behavior during extraction or purification.
Reagent manufacturers offer the compound in glass bottles, usually labeled well with product name, batch number, molecular formula, and purity ratings—often upwards of 98%. The CAS registry number stands as an unambiguous identifier for global regulatory scrutiny. Product sheets detail moisture sensitivity, recommended temperature ranges for storage, and necessary hazard warnings. Shipping requires UN-approved packaging since the presence of the acryloyl group can pose risks associated with acrylic monomers.
A common synthetic route involves acylation of morpholine using acryloyl chloride under carefully controlled basic conditions. One experienced chemist might prefer triethylamine as a base to trap the HCl byproduct, maintaining the pH for smooth amide formation. The reaction typically runs in anhydrous dichloromethane, offering both sufficient solubility for reactants and straightforward separation from salts and unreacted amines. After aqueous workup and column chromatography, the isolated product stands out for its purity. Those running industrial synthesis rely on continuous feeding and phase separation to scale up without generating problematic byproducts.
Researchers discovered the beauty of this compound in its double-sided potential: the acrylamide portion allows addition reactions, triggered by electron-deficient environments, and the morpholine ring serves as a nucleophile or base under the right push. Additions to the acryloyl double bond in combination with radical initiators or nucleophilic partners craft unique functionalized analogues. Crosslinking possibilities start opening up once you expose the compound to UV or heat, making it invaluable for hydrogel or coating development. Functional chemists often introduce further substitutions onto the morpholine ring to tailor water solubility or to append targeting ligands for bioactive materials.
Across catalogs, 4-(1-Oxo-2-Propenyl)-Morpholine sometimes appears as N-Acryloylmorpholine. Other references call it N-(2-Propenoyl)morpholine. Less precise suppliers simply list it as Acrylmorpholine. These synonyms often reflect specific regional markets or adopt the naming conventions of pharmaceutical or polymer industries. Check product sheets carefully since mislabeling can lead to confusion between acrylamide and acryloyl-morpholine derivatives.
Lab safety officers emphasize the need for gloves and goggles because the acryloyl group can sensitize skin and mucous membranes. The MSDS flags it as an irritant—accidental contact stings and may cause delayed dermatitis. The vapor pressure remains modest at ambient temperatures but rises with heating, so ventilation sits near the top of every checklist. Training teams run frequent drills on spill neutralization and waste handling, and industry settings require full air extraction at workstations. Glass storage over plastic is preferred to avoid trace contamination. Waste operators often deploy incineration in appropriate facilities to break down the stable amide linkage before landfill disposal.
This compound enjoys a loyal following among formulators of polymers, hydrogels, and adhesives. In biomedical engineering, 4-(1-Oxo-2-Propenyl)-Morpholine serves as a precursor for biocompatible polymers used in drug delivery—thanks to its ability to participate in mild, aqueous polymerizations. Dental materials manufacturers employ it for tough yet flexible resins, which resist brittleness under stress. Surface coating developers exploit its strong adhesion and controlled swelling properties in anti-fouling and anti-corrosive layers. Electronics always search for materials with reliable dielectric properties, so this molecule finds a niche in specialty encapsulants and adhesives for microchips.
Academic chemists and industrial labs both treat 4-(1-Oxo-2-Propenyl)-Morpholine as a canvas for new materials. Medicinal chemists investigate its role as a linker in targeted drug conjugates. Others embed it into responsive hydrogels that swell under specific pH or temperature triggers, boosting uses in soft robotics or diagnostics. Multi-institution collaborations examine ways to graft peptides or sugars onto the morpholine ring, seeking multifunctional biopolymers for tissue engineering.
Toxicologists study the compound in mammalian cell cultures and aquatic toxicity screens. Results to date suggest low acute toxicity, though irritant profiles do present concern for repeated skin or inhalational contact. Chronic exposure studies remain ongoing. Regulators in Europe and North America recommend gloves and fume extraction at minimum, with restricted volumes in teaching labs. The potential for breakdown into acrylamide analogues—a known neurotoxicant—drives conservative workplace limits. Researchers urge ongoing monitoring, especially as product volumes and end use grow.
Future application spaces keep growing as chemistry continues to diversify beyond legacy polymers. Biomaterials researchers anticipate further breakthroughs in targeted drug vehicles built on functionalized morpholine scaffolds. Electronics manufacturers express interest in biodegradable encapsulants as regulation tightens around e-waste. Regulatory frameworks will evolve, likely imposing stricter monitoring for occupational exposures and environmental release. Through all this, the core value of 4-(1-Oxo-2-Propenyl)-Morpholine—firmly rooted in reliable reactivity and flexible molecular design—will keep it in play across the chemical sciences, building on a foundation shaped by decades of lab experimentation and practical production knowhow.
4-(1-Oxo-2-Propenyl)-Morpholine doesn't make headlines, but it often plays a quiet role in products many people use every day. I first came across it during a late-night research session digging into food additives and packaging materials. Looking deeper, I found out this compound sits in the toolbox of chemists working on adhesives and food packaging.
Food safety agencies like the U.S. Food and Drug Administration (FDA) recognize the use of this compound in adhesives. Materials and coatings come in regular contact with food during packaging, transport, and storage. The EPA and European regulators track the chemicals allowed in these applications for a reason — a lot rides on the integrity and safety of what's being used near the food we eat.
Adhesives used in food packaging need to keep their seal without bleeding harmful ingredients into the food. The morpholine group provides chemical stability and resists breaking down under heat or pressure. It helps adhesives cling to surfaces — think of bottles, wrappers, and liners — and stand up to refrigeration or moisture. Acrolein-based structures, like the one found in 4-(1-Oxo-2-Propenyl)-Morpholine, allow fine-tuning of how sticky or strong the adhesive gets without letting unwanted stuff seep in. It’s not as eye-catching as marketing buzzwords, but this stability matters.
If you’ve ever peeled a stubborn sticker off a package, you’ve seen the handiwork of these adhesives. The sticky layer looks unassuming but goes through plenty of testing before regulators clear it for use around food. Under the Code of Federal Regulations (21 CFR 175.105), substances including 4-(1-Oxo-2-Propenyl)-Morpholine show up in the lists approved for indirect food contact, which means scientists closely monitor their residue levels to keep the exposure very low — a fraction of what your body naturally encounters every day.
Questions always come up about chemical residues. People want to know if their food picks up anything from wrappers or cans. Testing and regulations work together like a double lock. Agencies like the FDA only approve this sort of compound when they have solid toxicological data. They keep tabs on migration levels, so what's in the adhesive generally doesn't move into the food in any meaningful quantity. Over the years, safety standards have tightened as techniques for detecting tiny amounts of chemicals have advanced.
Sifting through public safety data, I see calls for more transparency and better science. Companies are under more pressure today to explain not just what goes in the package, but how it might interact with what’s inside. People expect not just regulatory compliance but also clean labels and fewer chemicals. The hope is that in the future, packaging materials will rely more on renewable resources and simpler chemistries — the sort that brings both function and peace of mind.
For now, substances such as 4-(1-Oxo-2-Propenyl)-Morpholine fill a narrow yet essential gap, creating safer, cleaner packaging for supermarket shelves. As scientists and public interest groups push for regular reviews and advances in safety testing, ongoing research offers real power to improve the balance between food preservation, transparency, and risk reduction.
Anyone who's spent time in a chemistry lab knows there's no room for letting your guard down. 4-(1-Oxo-2-Propenyl)-Morpholine—often used in industrial synthesis—doesn't look dangerous at first glance. But as an organic compound with reactive groups, it can sting your skin, bother your lungs, and even spark allergies if you're not careful. The Material Safety Data Sheets don’t sugarcoat: they tell you this chemical has serious bite. Even without a tragic story of chemical burns, the risk becomes real once you've handled similar substances and seen careless colleagues pay the price.
Without question, the most basic step involves dressing right. Long lab coats, chemical-resistant gloves, and safety goggles make up the standard kit. Face shields put up another layer, especially when splashes could reach your face. Once, I underestimated a similar compound and wound up with red, irritated skin that lingered for days. Since then, gloves and sleeves tucked tight have become non-negotiable.
Ventilation is something too many skip. Chemical vapors can sneak past weak fume hoods and cause real harm fast. Turning on that fume hood, checking the airflow (with actual tissue, not just a guess), and keeping containers tight makes it harder for gases and fumes to spread. I once worked in a poorly ventilated room, and just a couple hours there left my head throbbing and my throat sore. Fresh air and strong airflow make a difference you really feel.
Contamination spreads with just a swipe of the hand. Before lunch breaks and after shifts, thorough washing with soap cuts down on anything that may linger. Never taking lab gloves outside the work zone keeps home and public areas safe. Designating lab zones works well; spills outside those spaces turn up more surprises than anyone wants.
Some folks keep open coffee mugs or snacks on crowded benches. I've seen spills splash into personal items, causing everything from ruined datasheets to accidental exposure. Keeping food and drink far from the action isn't just a best practice. It's the kind of habit that keeps everyone healthier.
Spills sometimes happen, even when nobody wants them to. That's where absorbent pads, neutralizing agents, and proper disposal containers step in. Having a spill kit within reach, and actually rehearsing how to use it with your team, brings a bit of peace of mind. In one situation, a quick-thinking colleague tossed down the right absorbent material before a spill reached electrical wiring—potentially stopping a full-blown disaster.
Waste disposal routines don’t get enough attention. Dumping chemicals down the drain infects water and lands an operator in heaps of regulatory trouble. Labeled hazardous waste bins, regular pickups, and keeping logs shield workers and the environment from the fallout.
No list of safety steps replaces clear discussion and frequent drills. Reading the most recent safety data sheets, keeping emergency contacts visible, and running practice evacuations help teams act quickly in a crisis. Younger lab members often pick up safe behaviors by watching more experienced coworkers. Clear signage, honest conversations about risks, and responsive supervisors shape a culture where everyone steps up.
Backed by regular reviews and attention to detail, simple routines reduce both small mishaps and major accidents. After years in the lab and industry, it's clear that sticking to these habits gives you a better shot at a safe shift and a healthy career.
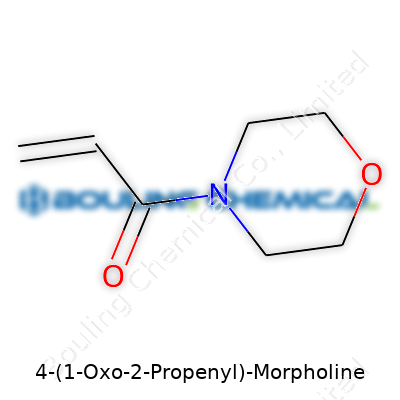
Organic chemistry names can sound like puzzles, but most have clues that point straight to the structure. The name “4-(1-Oxo-2-Propenyl)-Morpholine” sketches a clear blueprint. Start with morpholine—a six-membered ring featuring both oxygen and nitrogen. The “4-” whispers the location: the collection of extra atoms latches on to the fourth spot of this ring. This ring pops up in pharmaceuticals, crop science, and polymer research because both the oxygen and nitrogen boost reactivity and binding strength.
Morpholine on its own doesn’t stir much excitement, but the “1-Oxo-2-propenyl” group spices things up. That phrase points to an acrolein-like tail (CH₂=CH–CO–). Directly from the ring, you have a three-carbon side chain. It’s not just a dull line of atoms—the oxygen doubles up as a carbonyl at the first carbon, then a double-bond between the central carbon and the last carbon. So, you end up with CH₂=CH–CO– connected to morpholine’s fourth position.
Linking it all together, you see a morpholine ring, with the “tail” at carbon four. Draw it out, and you spot the nitrogen opposite the oxygen in the ring, and the side chain jutting out, carbon by carbon. Anyone with organic chemistry in their background will recognize this signature arrangement: a heterocyclic ring with a reactive alpha,beta-unsaturated carbonyl side chain.
Back during graduate school, I spent hours mapping out molecular frameworks and comparing how small changes in side chains ramped up or toned down function. This molecule’s design calls out to those same instincts. That carbonyl group next to a double bond (alpha, beta-unsaturated carbonyl) creates a reactive hot spot. Such setups eagerly engage in Michael addition reactions—a chemistry workhorse for building larger, more complex molecules. Medicinal chemists recognized years ago that this kind of motif speeds molecular shuffling, kicking off chain reactions useful for blocking enzymes or latching onto troublemaking free radicals.
The interplay between morpholine and an acrolein-like moiety isn’t just an academic curiosity. A lot of agricultural fungicides, antioxidants, and blood pressure meds feature similar ring structures and side chains. Researchers use these components to engineer better precision, stability, or bioavailability—traits that decide whether a substance can actually help solve real-life health challenges. Scientists with years in the lab can spot the hidden power inside a simple diagram: the right side chain can make all the difference, flipping a molecule from inert to indispensable.
Running chemistry with acrolein-type compounds isn’t a walk in the park. Anyone with lab time knows this group is feisty—irritating to skin and lungs, eager to polymerize or oxidize if left unchecked. Researchers often wrestle with containment, turning to safer analogues or controlled conditions. Swapping these functionalities into a stable ring like morpholine helps tame that reactivity, letting chemists nudge the molecule into performing work without blowing up lab safety protocols. Strict ventilation, sealed vessels, and protective gear are daily practice with molecules like this for a reason.
Building safer, more versatile molecules begins here. Teams now look for ways to tweak the morpholine head and the reactive tail, focusing on precision design. By adding bulkier side chains or integrating protective groups, the risk of runaway reactions drops. Collaborations bridging industrial, pharmaceutical, and academic labs keep the focus on sustainability—finding smarter catalysts, recycling solvents, and moving toward greener chemistry. The structure of 4-(1-Oxo-2-Propenyl)-Morpholine stands not just as a textbook example, but as a reminder that chemical ingenuity can open doors that once seemed sealed.
Working in labs over the years, I’ve learned that chemical safety doesn’t start with the experiment. It starts much earlier, in the ways we store what we use every day. Take 4-(1-Oxo-2-Propenyl)-Morpholine. Anyone who checks its datasheet understands it can’t just sit anywhere. You need a steady approach and discipline, or the risks climb real fast.
This compound, like many reactive organics, reacts badly with moisture and high heat. In one of my roles, I saw a technician handle a similar compound in a storeroom with a busted air conditioner. Within a week, containers caught sweat marks, labels curled, and more than one bottle ruined before anyone realized. Moisture isn’t just an inconvenience. It can trigger slow decomposition, releasing irritant fumes or even creating unexpected byproducts.
Heat ramps up the problem. Left near equipment vents or sunlit windows, bottle pressure can spike. I’ve seen caps start bulging, turning simple errors into emergencies. So, environmental control isn’t about box-ticking. It’s about removing surprises. Reliable storage habits cut the risk of accidents and wasted chemicals—both hurting time and budgets.
A dry closet or chemical fridge outperforms fancy smart sensors every time if monitored right. Avoid storing this compound near acids, bases, or reactive metals. Cross-contamination turns small mistakes into chain reactions. Label shelves, physically separate incompatible materials, and take five seconds to double-check before shelving anything.
Light plays its own role in chemical stability. Leaving bottles exposed to fluorescent or direct sunlight breaks bonds inside the compound, leading to slow breakdown. Amber glass bottles and solid cupboard doors help block out light on a very practical level.
Locking storage isn’t just bureaucratic hassle. Accidents don’t always come from dramatic spills—sometimes someone grabs the wrong bottle in a rush. Secure access, proper signage, and discipline with check-in/check-out logs go a long way in busy spaces.
Sometimes you find old bottles with crusted lids or funny smells. Don’t shrug these off. I had a colleague who tried to clean up an old spill without gloves or respirator—he didn’t go home that day. Good ventilation isn’t about fresh air alone; it’s about carrying away fumes before they become a problem. Flammable storage cabinets with built-in exhaust systems are worth every penny in shared environments.
Spill kits belong as close as the bottle itself. Absorbents, gloves, goggles, and disposal bags should never collect dust. People skip these steps, thinking it’s all just downtime. It only takes one incident to prove how wrong that thinking runs.
Teach storage rules on day one, and reinforce them often. Laminated placards, color-coded tags, and quick safety talks make a bigger difference than any thick manual. Fact is, most lab accidents stem from small lapses. Keeping 4-(1-Oxo-2-Propenyl)-Morpholine safe boils down to teamwork, vigilance, and respect for procedures—each grounded in real-world experience, not just theory.
Routine inspections guard against hidden leaks and label fading. Rotate stock, keep a tight inventory, and never skimp on secondary containment. Double-check everything, even if it seems fine. Mistakes multiply when you grab and go without looking.
Manufacturers provide guidelines, but adapting those to real-world spaces makes the biggest difference. Every person who walks into a storeroom or lab space holds a piece of the safety puzzle. Share stories, review procedures, and make room for honest questions from everyone—new hires to veterans. That’s the kind of culture that protects both people and productivity.
When talking about chemicals like 4-(1-Oxo-2-Propenyl)-Morpholine, every percent in purity makes a difference. Researchers and quality control teams often check on standards such as “not less than 98%” as set both by in-house and international pharmacopeia norms. The target here is always a clean product with minimal impurities. Impurities above 2% are usually red flags, because those traces can introduce all sorts of headaches—from skewed research data to genuine safety concerns in pharmaceutical development.
It’s not just about ticking boxes or pouring clear liquids into beakers. Everything from dosage form design in drug development to chemical synthesis in materials science relies on reliable input. Skipping the purity check can mean walking blindfolded. I once saw a batch of intermediates rejected simply because a reagent didn’t meet the published specs. Someone assumed the “gray area” would make no difference. The benchwork told a different story: low yields, repeat runs, and wasted resources.
For 4-(1-Oxo-2-Propenyl)-Morpholine, manufacturers usually specify purity by weight percentage, moisture content, and limits on related substances or residual solvents. Typical guidelines call for at least 98%, with moisture not surpassing 0.5%, and individual related impurities below 0.5%. You’ll see these numbers on Certificate of Analysis sheets from responsible suppliers. Strong protocols in gas chromatography and HPLC screening help spot even those tiny contaminants that may slip through a routine check. Documentation on elemental analysis, residual solvents (such as toluene or methanol under 100 ppm), and endotoxin content support confidence in lab conditions and human safety.
Chemists and pharmacists notice even minor impurities. These can react with active pharmaceutical ingredients, cause side reactions, or compromise shelf life. Exposure to unexpected byproducts risks forming toxic metabolites. That’s not a hypothetical; it’s a well-known risk from decades of medicinal chemistry failures. Defining clear specs helps avoid those pitfalls. I learned early in my lab days to ask for an updated analysis whenever a new lot arrived—in-house retesting sometimes found batches below spec, even from trusted names.
Sometimes a manufacturer’s published spec sits above 98%, but specific projects demand 99% or better for sensitive bioassays or analytical reference materials. That’s where purification steps like recrystallization, distillation, or preparative chromatography come in. These add cost and time, but they can save a project from much bigger failures. Auditing suppliers matters too. Third-party labs sometimes find problems that company inspectors miss. Regulatory agencies, especially in pharmaceuticals, don’t shrug off out-of-spec deliveries, and neither should any serious research program.
Industry-wide, constant improvement in analytical techniques narrows the risk margin. Real expertise means knowing how to interpret a mass spec readout, understanding regulatory language, and asking the tough questions about storage and handling. Too much humidity in storage rooms, old containers, or lack of inert atmosphere can degrade even a high-purity batch. In some cases, batch-to-batch consistency outranks a single sparkly purity number. Companies that invest in full traceability and transparency signal reliability. So for anyone specifying or buying 4-(1-Oxo-2-Propenyl)-Morpholine, always check purity specs closely, and back up your choice with regular independent testing.

| Names | |
| Preferred IUPAC name | 4-(Prop-2-enoyl)morpholine |
| Other names |
4-(1-Oxo-2-propenyl)morpholine N-Acryloylmorpholine Acrylomorpholine Morpholine, 4-(1-oxo-2-propenyl)- 4-(Acryloyl)morpholine |
| Pronunciation | /ˈfɔːr wʌn ˈɒk.səʊ tuː prəˈpiːnɪl ˈmɔː.fəˌliːn/ |
| Identifiers | |
| CAS Number | 6602-96-0 |
| 3D model (JSmol) | `3D structure; JSmol string for 4-(1-Oxo-2-Propenyl)-Morpholine`: `C=CC(=O)N1CCOCC1` |
| Beilstein Reference | 1093876 |
| ChEBI | CHEBI:189278 |
| ChEMBL | CHEMBL2106656 |
| ChemSpider | 21544116 |
| DrugBank | DB08292 |
| ECHA InfoCard | ECHA InfoCard: 100.041.920 |
| EC Number | 619-541-1 |
| Gmelin Reference | Gmelin 99723 |
| KEGG | C09714 |
| MeSH | C085416 |
| PubChem CID | 67803 |
| RTECS number | UY4375000 |
| UNII | XO4QH3U9B4 |
| UN number | 3077 |
| CompTox Dashboard (EPA) | DTXSID90115928 |
| Properties | |
| Chemical formula | C7H11NO2 |
| Molar mass | 155.18 g/mol |
| Appearance | Light yellow liquid |
| Odor | Odor: characteristic |
| Density | 1.112 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | 0.02 |
| Vapor pressure | 0.01 mmHg (25 °C) |
| Acidity (pKa) | 5.2 |
| Basicity (pKb) | 6.89 |
| Magnetic susceptibility (χ) | -57.85×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.529 |
| Viscosity | Viscosity: 35.2 mPa·s |
| Dipole moment | 2.99 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 373.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4512 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | N02AC11 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | Precautionary statements: P280, P305+P351+P338, P302+P352, P332+P313 |
| NFPA 704 (fire diamond) | 2-1-1 |
| Flash point | 88 °C |
| Lethal dose or concentration | LD₅₀ oral rat 590 mg/kg |
| LD50 (median dose) | LD50 (median dose): 238 mg/kg (oral, rat) |
| NIOSH | MU8575000 |
| PEL (Permissible) | PEL (Permissible exposure limit) for 4-(1-Oxo-2-Propenyl)-Morpholine: Not established |
| REL (Recommended) | 1 mg/m^3 |
| Related compounds | |
| Related compounds |
Acrylamide Morpholine N-(2-Hydroxyethyl)acrylamide N-Methylacrylamide |