Decades ago, laboratories chasing novel heterocycles stumbled upon the piperazinone scaffold. Chemists realized that the addition of a methyl group at the 3-position of piperazin-2-one creates a new compound, (3R)-3-Methylpiperazin-2-One, which brought possibilities across pharmaceutical research. The journey didn't start with grand ambitions—this molecule emerged from iterative synthesis and the old curiosity to tweak, test, and learn. As researchers developed routes with tighter stereocontrol, access to the (3R)-enantiomer opened doors to chiral complexity, and with it, new applications in medicinal chemistry.
This compound serves as a chiral building block in discovery pipelines. Its structure, featuring a rigid lactam ring and a methyl at the 3-position, makes it attractive for those searching to increase the three-dimensionality of a candidate drug. Pharmaceutical developers often describe it as a bridge between routine heterocycles and more challenging architectures required for next-generation therapeutic agents. Its ready reactivity and relatively simple handling set it apart from more finicky intermediates.
(3R)-3-Methylpiperazin-2-One typically appears as an off-white solid—powdery, sometimes a bit sticky in humid labs. Chemists have clocked its melting point at around 95–99°C, a range confirming purity and structure after each batch is made. This compound dissolves easily in DMSO and methanol, making it practical for reaction development and scale-up. Its reactivity revolves around the lactam carbonyl and the secondary amines, which show predictable behavior in both mild and robust chemistries. Chemical stability holds up under regular storage, but exposure to excess heat can degrade it, which anyone who's left a batch on a hotplate for too long has learned firsthand.
Reputable suppliers provide material with optical purity above 98%, and impurities rarely top 1%. Labels highlight the enantiomeric configuration, batch number, and analytical data like 1H NMR spectra and HPLC retention times. These details serve more than bureaucratic precision. Projects in the trenches depend on knowing purity and origin—simple documentation, once overlooked, now forms the backbone of compliance, reproducibility, and efficient troubleshooting.
Typical laboratory syntheses begin with a protected piperazinone precursor. Enantioselective alkylation enters the picture, often using a chiral auxiliary or asymmetric catalyst to steer the methyl group to the right spot. Some research teams rely on enzymatic methods to increase selectivity, finding cost balances as batch sizes scale. Each route must consider not only yield but also waste handling and recovery of costly reagents. In scale-up, access to cheaper starting materials and step economy decides if the process leaves the lab or stays on paper. The switch from bench to pilot plant means working with real-world solvents, not only what’s convenient in a fume hood. The move away from legacy approaches—those relying on excessive chromatography—has pushed greener, more robust options into the spotlight, making batch-to-batch consistency attainable.
Functionalizing (3R)-3-Methylpiperazin-2-One doesn’t require extreme conditions or rare reagents. The ring tolerates reductive aminations, N-alkylations, and acylations, expanding the utility into various chemical libraries. Skilled chemists have used both traditional and microwave-assisted protocols to quickly screen analogs, shaving weeks off timelines in the hit-to-lead stage. In med chem settings, introducing substituents at the nitrogens or building sp^3-rich appendages is standard practice, each mod shaping solubility and target interaction profiles. The lactam motif plays a dual role—robust in drug metabolism studies and pliable under controlled hydrolysis or reduction. Newer research often explores cross-coupling at the C3 methyl group, bringing more diversity to scaffold designs—a feature anyone with a tight SAR deadline can appreciate.
Across catalogs and articles, this compound hides behind several names. Some call it (R)-3-Methyl-2-piperazinone, others 3R-Methylpiperazinone-2. Chemical vendors sometimes list it under protected forms or as intermediates for larger syntheses. The key is reference to the (3R) chiral center and 2-one functionality. Recognizing these synonyms prevents the confusion that once led many a purchasing manager to order the wrong enantiomer, disrupting weeks of work.
Safety data sheets flag this compound as a mild irritant. Regular lab hygiene—gloves, goggles, and working under a fume hood—cuts down risk. Spills clean up with standard absorbent material, but those who overlook ventilation can expect mild headaches or throat irritation after an afternoon at the bench. In bulk, storage practices keep the container sealed, at low humidity, away from oxidants. Shipping regulations treat it as non-hazardous, but responsible sites still file risk assessments and retain batch analysis on file, which matters when audits roll in. Waste management stays straightforward: water-diluted washes go to organic collection, and the main environmental concern focuses on preventing runoff into regular drains.
This compound shows up most often in pharmaceutical research, especially for those building new CNS-active molecules or looking to optimize drug-like properties. Chemical biology leverages the rigidity and chiral center for probe development. Agrochemicals researchers also find value here, tweaking pesticidal backbones for selective toxicity. Anyone navigating the lead optimization maze appreciates the scaffold for its functional handle and the metabolic stability it lends to core structures. Water solubility, decent oral bioavailability, and recognition in high-throughput screens have secured it a reliable place in many synthetic chemists’ toolkits. Medicinal chemistry teams often slot it into the early stages of SAR exploration, pushing boundaries in potency and selectivity—sometimes finding success, sometimes returning to the drawing board with a sharper focus.
Early days saw (3R)-3-Methylpiperazin-2-One relegated to side-project status, but now well-funded collaborations make heavy use of it. Automation and high-throughput experimentation have taken root, pushing the need for larger, more consistent batches. Synthetic chemists at contract research organizations keep refining access routes, looking to trim costs and simplify work-ups. Academic groups chase novel transformations, boosting yields or greening up the process. Teams have started to broach machine learning, aggregating reaction data to find pattern recognition—speeding route optimization. The focus isn’t just on scale, but reproducibility—essential in this era of global partnership and regulatory pressure.
In-depth toxicology remains relatively sparse for the parent molecule, but early screens point to a low acute toxicity profile in rodents at relevant therapeutic doses. Chronic studies, hepatotoxicity, and long-term bioaccumulation haven’t raised major flags, but gaps still exist. Some metabolic byproducts linger in microsomal incubations longer than ideal, sparking questions about possible tissue retention. Environmental teams push for more data on breakdown products, especially as industrial use grows. Anyone who’s navigated the minefield of regulatory submission knows early toxicity screening saves time and money long before candidates hit animal studies. Academic consortia now look to fill in these gaps, accelerating with advanced in vitro technologies and digital models—providing much needed, real-world relevant information.
Growth in personalized medicine and targeted therapies will likely increase demand for specialized chiral intermediates such as (3R)-3-Methylpiperazin-2-One. Diversification of synthetic methods—with renewed interest in asymmetric catalysis and renewable feedstocks—should make its production greener and more cost-effective. Companies focused on small-molecule therapeutics bank on this scaffold for novel kinase inhibitors, ion channel modulators, and antiviral projects. Regulatory expectations for traceability, documentation, and safety will keep sharpening production standards. Researchers exploring AI-driven drug design may widen the application further, as this motif adapts well to structural diversity required by new algorithms. As chemical supply lines mature, expect this once-obscure intermediate to become a staple in both R&D labs and scaled manufacturing, shaping the future of drug and agrochemical discovery across the globe.
(3R)-3-Methylpiperazin-2-One sounds obscure, sure, but chemicals like this shape many medicines that keep people alive and healthy. You probably won't read about it on a pill bottle from the pharmacy, yet researchers and pharmaceutical scientists work with compounds like this every day. I’ve spent my share of hours reading technical papers and talking to chemists. I know how crucial these molecules become when building drugs that target illnesses most people can't even pronounce.
This chemical acts as a core building block for creating new pharmaceuticals. It helps experts piece together more complex drugs by serving as a starting piece — imagine it like a puzzle corner that brings everything else together. Many companies rely on it during drug discovery phases, particularly for medications that deal with the central nervous system or bacterial infections.
Medicinal chemists often look for ways to tweak molecules so they can deliver better results while avoiding unwanted side effects. (3R)-3-Methylpiperazin-2-One lends a hand here. Its specific structure helps fine-tune activity at biological targets. Chemists often use it when developing compounds that need to fit just right inside certain receptor pockets in the body. Adjusting a single part of a molecule can change everything about how a drug works — it can mean the difference between relief and a missed opportunity.
What makes this chemical especially relevant is its chiral purity. Plenty of drugs work best when they're made with right- or left-handed versions — much like your shoes only fit on the correct foot. Making sure you have the correct version can cut down risks and boost how well a medicine does its job. Managing this detail can save both time and money in the development pipeline, which matters to anyone waiting for better treatments.
Labs and companies that work with antibiotics, antiviral drugs, or even breakthroughs in oncology, often turn to specialized molecules like this. Adjusting small rings and structures boosts a drug’s penetration, stability, or even helps it dodge resistance in bacteria — a real headache in today’s health care landscape. Innovation stems from mixing practicality with creativity, and that process depends heavily on these fundamental building blocks.
Availability and cost challenge small labs and big firms alike. Purity standards must run high. Any contaminant in the batch can mess up research results or trigger nasty reactions in clinical tests. The focus on ethical sourcing and eco-friendly production keeps growing. Some suppliers have started exploring green chemistry routes, which use less toxic materials and waste fewer resources.
Training the next generation of chemists to handle these intermediates safely also grows more urgent. Mistakes in the lab cost time, trust, and sometimes more. Companies and universities can help by providing hands-on experience and clear guidelines. This chemical, like many others, won’t solve medical crises on its own, but it’s an unmistakable part of the path forward.
(3R)-3-Methylpiperazin-2-One may sound like science fiction, but everyday improvements in medicine trace back to substances like this. Progress takes more than big headlines. It depends on unseen work and the reliable pieces that build a future where more people get the help they need.
Chemistry names look complicated, but each part tells a story about the molecule. Take (3R)-3-Methylpiperazin-2-one. This compound isn’t something you bump into unless you work or study compounds in pharmaceuticals or research labs. The trick is to read the name step by step, figure out the structure, and see what the formula says about its properties and uses.
Piperazine forms the basic skeleton: a six-membered ring with two nitrogen atoms. These nitrogens sit opposite each other, which impacts how the molecule behaves. A methyl group hangs off the third carbon, and there’s a ketone at the second position. That “(3R)” tells you which way the methyl group points—a little detail packed with meaning for researchers working on drug development or chemical engineering.
Count the atoms: the backbone holds four carbons, two nitrogens, then you add in the methyl group on carbon 3, a single oxygen for the ketone. Put this together, you get C5H10N2O. Each atom in this formula plays a role. The carbons shape the ring, the nitrogens open up paths for hydrogen bonding, and the oxygen lets the compound take part in reactions that plain piperazine can’t touch.
Misreading a molecular formula in the lab can lead to costly mistakes. Maybe you make a batch with the wrong configuration, or miss out on a promising result because the chirality is off. Personal experience tells me precision with naming and notation stops headaches down the road. Pharmaceutical synthesis, for instance, relies on getting chirality right—(3R)-3-Methylpiperazin-2-one has a specific three-dimensional shape, and in medicine, one enantiomer might help while another could do harm.
During college, a misdrawn formula on the whiteboard for another piperazine derivative sent our whole lab group off on a wild goose chase, burning up resources for days. Cross-checking the chemical name against the structure would’ve saved time. Mistakes like this push home the value of careful analysis. They underline how every subscript and prefix in chemistry brings a set of properties—solubility changes, reaction rates shift, biological effects swing. Even one atom out of place, and you get a different compound.
Many people using chemicals for drug discovery, manufacturing, or agrochemical development count on precise formulas. Patents live and die on details like the orientation of a methyl group. Technology helps—software can check chemical names and spit out structures in seconds. Still, knowing how to double check that C5H10N2O matches (3R)-3-Methylpiperazin-2-one keeps things running smoothly and backs up digital tools.
Education matters. More time spent in organic chemistry courses, drawing out structures by hand, creates a second nature understanding you won’t get from relying only on automatic tools. Building this habit stops errors, especially during scale-up or sharing results with regulatory agencies who check everything twice.
Understanding the chemical formula of even a niche compound like (3R)-3-Methylpiperazin-2-one means more than reciting C5H10N2O. It involves grasping how structure and stereochemistry thread into the backbone of real-life applications, product safety, and research success. Getting this right makes science and industry run better for everyone.
(3R)-3-Methylpiperazin-2-One stands out as a specialized chemical, often showing up in research labs and development environments. In my time navigating lab safety protocols, I learned the hard way that ignoring basic storage advice quickly leads to compromised samples and potential hazards. The chemical’s molecular structure tells us it’s hygroscopic—it loves to draw water out of any environment. Even a couple of hours exposed to humid air can turn a high-quality sample into something unreliable or even unusable.
Mixing chemicals during a muggy summer back in graduate school, someone left a sample of a similar piperazine derivative on an open shelf. Later analysis showed the compound had degraded, ruining the entire batch and slowing down everything we planned. For this reason, (3R)-3-Methylpiperazin-2-One does best in a cool, stable climate. Temperatures between 2°C and 8°C work well. A standard laboratory refrigerator usually delivers exactly this range. Sticking the container into a freezer isn’t necessary, and it can risk condensation that leads to moisture-related problems. Consistency beats subzero extremes here.
Anyone who’s handled this substance over a few months can spot the difference between material stored in an airtight vial and a forgotten, loosely capped bottle. Exposure to the air means unintended chemical reactions, sometimes just enough to affect purity, sometimes much worse. Always choosing containers with a tight seal—borosilicate vials with screw-top lids or PTFE-lined closures—helps guard against these threats. Add a desiccant packet for further insurance, especially in environments with unpredictable humidity. Even the best-designed cap won’t solve the moisture problem written into the chemical’s nature, so every additional protection counts.
Fluorescent light in a lab often emits wavelengths that cause degradation over weeks or months. Amber glass containers filter out UV and visible light, reducing the risk. Keep the vial tucked in a drawer or storage cabinet that stays dark unless opened. Alongside protection from light, labeling every container matters more than most realize. I’ve seen accidents result from mislabeling or faded markers, where someone assumed they were using another reagent entirely. Proper labels state the chemical name, concentration, date received, and expiry or retest date.
Every staff member working with (3R)-3-Methylpiperazin-2-One should know the company’s storage rules inside and out. Posting a chemical hygiene plan near the storage area sets the expectation for anyone grabbing a sample. Add a safety data sheet (SDS) within reach. If a spill or degradation does happen, clear instructions mean there’s no guessing or panicking—just a practiced response. Restoring order becomes an ordinary act, not a crisis.
Ordering only the quantities needed for immediate projects limits the risk of deterioration. Purchasing in smaller lots ensures that material used up sooner. Disposing of leftovers before they go stale reduces the buildup of old, questionable stock. Auditing inventory every few months catches problems before they become disasters.
Reliable results in research rely on careful routines. By storing (3R)-3-Methylpiperazin-2-One in a clean, cool, dark, and dry place in a sealed container, mistakes shrink and efficiency rises. Each invested moment up front saves time, money, and headaches down the line. Good habits grow best in teams that value consistency and care at every step.
Anyone tracking the world of fine chemicals has probably noticed the growing attention on (3R)-3-Methylpiperazin-2-One. In my years of following specialty compounds and talking to buyers and suppliers, I’ve learned a few truths about what “available in bulk” really means. There’s no shortcut to honest sourcing, so it’s worth digging into what drives supply for this molecule and how labs or manufacturers can actually secure large amounts of it.
This compound often pops up in pharmaceutical research, agrochemical development, and advanced chemical synthesis. Labs need more than a few grams—usually hundreds of grams, kilos, sometimes much more. Bulk demand comes from the scale-up phase, where bench-scale reactions shift into runs big enough for animal studies or commercial development. No research moves forward without reliable, repeatable access to bulk product. That’s not just a convenience—it’s make or break for whole projects.
Securing a steady flow starts with capacity at the manufacturing site. In my experience, most of the global stock of (3R)-3-Methylpiperazin-2-One doesn’t sit waiting on a warehouse shelf. Most suppliers produce it to order, with timelines tied to raw material access, batch scheduling, and regulatory hurdles. China and India host several large producers, but European and US firms sometimes carry custom synthesis capabilities. Big output relies on experienced chemists, pollution control, and reliable logistics networks.
Requests usually trigger a back-and-forth with sales managers—expected delivery, price per kilo, documentation, and required purity all factor in. Some suppliers lose out because they can’t hit requested specs, others quote lead times that stretch into months. A supplier may claim “bulk available,” but real-world checks — phone calls, quotations, and site visits — paint a more complex picture. In practice, only a handful can pull off timely 25 kg, 50 kg, or 100 kg runs with supporting paperwork. Cutting corners isn’t an option for firms facing audit inspections or regulatory checks.
In the current market, I never take a bulk offer at face value. I always ask for factory audit reports, regulatory compliance records, batch COAs (Certificates of Analysis), and previous shipment history. Bulk buyers—especially from regulated industries—should check not only technical specs but also environmental and safety records. Recalls and failed deliveries slow whole pipelines. Sites like ChemSpider or PubChem list the chemical, but only active outreach extracts actual stock info. Vetting options through industry forums, trade shows, and even LinkedIn saves headaches down the line.
If suppliers want to meet surging demand, upgrades on process safety, documentation transparency, and customer communication will help. Open discussions about timelines and bottlenecks build trust. Sharing real-time production schedules, offering reference checks, and allowing third-party audits give buyers the confidence they need for major purchases. Creating better pre-shipment sampling and third-party lab testing eases concerns about consistency—nobody wants to pay for 20 kilos of off-spec material.
Real-world experience shows the road from lab-scale to bulk purchase is littered with miscommunications, empty promises, and false starts. Still, plenty of legitimate suppliers can scale (3R)-3-Methylpiperazin-2-One production if approached with the right information and fair expectations. Only old-fashioned due diligence—references, documentation, background checks—brings confidence to the deal.
Working with (3R)-3-Methylpiperazin-2-One isn’t just a numbers game. A lab tech might look at a purity grade—maybe 98% or 99%—like it’s just a label, but there’s a lot wrapped up in that figure. High purity means fewer headaches: no hidden peaks scrambling results, no downstream side reactions to untangle. In organic synthesis, a compound that’s off by half a percent transforms a routine process into a troubleshooting saga. Purity at 98% usually covers standard pharmaceutical research, but specialty applications ask for HPLC, GC, and even NMR traceability to back up that claim.
Every chemist has that story: an off-batch throws the research off for weeks. Contaminants—tiny amounts that the eye can’t spot—start to show up in late-stage analytics. A company might publish their grade as “98% by HPLC,” but the real test is in the application. Unwanted peaks in a chromatogram often tie back to a synthesis step or storage problem, and those impurities dampen the reliability of experiments or manufacturing. That’s why most researchers request supporting documents. A single misstep in a bioactive project ripples through regulatory paperwork and can even trigger recall cycles if not caught early.
Buying chemistry raw materials has changed. It used to be enough to see “analytical grade” and take it at face value. Now, most companies ask for a full certificate of analysis, spectra, stability data, and a paper trail for every bottle. A good supplier shows all this upfront, reducing the back and forth. Trust, in this case, has roots in repeated orders and clear answers on every lot. Documentation isn’t just bureaucracy; it stops small mistakes before they cost real money or reputation.
Beyond one experiment or production run, consistency in purity shapes the growth of applied chemistry fields. Take drug development—where the margin for error is razor-thin—or advanced materials work, where low-level metals or unreacted byproducts cause device failures. Companies invest in source verification and third-party testing because those costs pale next to a failed approval or product recall. Real progress in drug discovery or battery chemistry often comes from minimizing variables, so top-shelf purity acts like a foundation for more reliable research.
Progress isn’t only about cleaner labs or faster analytics. Purity improvements rely on better synthetic routes, smarter purification steps, and honest reporting from suppliers. Some labs collaborate directly with producers to optimize upstream reactions and control batch-to-batch variation. Colleagues who dig in with suppliers, talk through synthetic routes, and invest in routine QC find their projects progress faster. Open dialogues about methods and standards cut down confusion over results and wasted time on quality concerns.
In everyday work, people find success by not only asking for high purity but also demanding transparency and consistency. Lab managers keep a file of trusted suppliers, tracking every hiccup and every win. It’s rarely flashy, but most breakthroughs rest on carefully sourced materials and a keen eye for the details hidden in purity grades. Not all purity claims are created equal, so putting effort into verification and partnership with suppliers sidesteps obstacles later on.
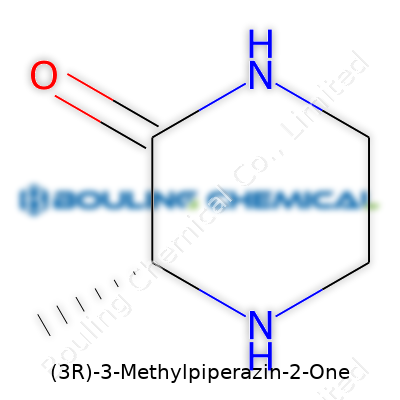
| Names | |
| Preferred IUPAC name | (3R)-3-methylpiperazin-2-one |
| Pronunciation | /ˈθriː-ɑːr θriː-ˈmɛθɪl.paɪˈpɛrəˌziːn-tuː-oʊn/ |
| Identifiers | |
| CAS Number | 41661-47-6 |
| 3D model (JSmol) | `3Dmol.data('C1[C@H](NCCN1)C(=O)N').setFormat('smiles')` |
| Beilstein Reference | 131253 |
| ChEBI | CHEBI:143307 |
| ChEMBL | CHEMBL3184749 |
| ChemSpider | 22134780 |
| DrugBank | DB08363 |
| ECHA InfoCard | 03b8c1d3-2e90-428b-892a-ad1166bee1cf |
| EC Number | 87673-69-6 |
| Gmelin Reference | 1823074 |
| KEGG | C21130 |
| MeSH | D059325 |
| PubChem CID | 101537892 |
| RTECS number | UG8245000 |
| UNII | A7D8X5T7PS |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C5H10N2O |
| Molar mass | 114.16 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.08 g/cm³ |
| Solubility in water | soluble |
| log P | -1.1 |
| Vapor pressure | 1.06E-2 mmHg at 25°C |
| Acidity (pKa) | pKa = 8.62 |
| Basicity (pKb) | 3.97 |
| Magnetic susceptibility (χ) | -11.31·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.547 |
| Dipole moment | 3.49 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 333.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -120.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4277.1 kJ/mol |
| Pharmacology | |
| ATC code | N06AX18 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06,GHS08 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P261, P264, P271, P280, P304+P340, P305+P351+P338, P312, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | Flash point: 207.2 °C |
| NIOSH | RZ2985000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for (3R)-3-Methylpiperazin-2-One is not specifically established by OSHA or other major regulatory agencies. |
| REL (Recommended) | 0.05 ppm |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
2-Methylpiperazine Piperazin-2-one 3-Methylpiperidine 3,6-Dimethylpiperazine N-Methylpiperazine |