Research circles started paying attention to 1,2-benzothiazole frameworks decades ago. Their biological activity struck a chord with chemists across Europe and Asia looking for new approaches to pharmacology and materials science. The addition of a piperazine ring at the 3-position was more than a footnote—labs saw higher selectivity for certain central nervous system receptors, which led to a wave of late 20th-century publications and patents. Synthetic tweaks over the years connected this scaffold to antipsychotic agents and antiparasitic experiments, while the ease of introducing different substituents made it a favorite for medicinal chemistry. From a historical lens, the route to 3-(piperazin-1-yl)-1,2-benzothiazole captures the shift from broad-spectrum screening to engineered, purpose-built small molecule drugs.
3-(Piperazin-1-yl)-1,2-benzothiazole stands out because of its dual nature: the benzothiazole lends rigidity, and the piperazine brings flexibility for binding in biological targets. This hybrid often provides a launching pad for brain-penetrant drug development, serving as a base for antipsychotics, antimalarial prototypes, and more. Pharmas and research groups value this compound’s role as a versatile intermediate. Its skeleton adapts to a broad set of modifications, opening a toolkit for a range of end uses beyond just drug development—think fluorescent probes, dye chemistry, materials for electronics, and even agricultural technologies. Each specific derivative brings its own twist, but the 3-(piperazin-1-yl) substitution stays a reliable workhorse in the chemical lineup.
Pure samples of 3-(piperazin-1-yl)-1,2-benzothiazole usually show up as off-white to pale yellow crystalline powders. Solid at room temperature, with moderate solubility in polar organic solvents like DMSO, DMF, or even dilute acids, it gives chemists flexibility in setting up reactions and analysis. Melting points often fall in the range of 170-210°C, a handy detail for purification by recrystallization. The presence of the piperazine influences basicity, so the compound’s solutions often lean alkaline. Its UV absorption and unique NMR spectra stand out, letting researchers easily distinguish it during purity checks or structure confirmations.
Any supplier worth their salt provides this molecule with documentation listing assay purity, usually topping 98%, alongside analytical data like HPLC, MS, and NMR profiles. Product sheets also call out key batch details, impurity thresholds, and sometimes residual solvent analysis, depending on the intended use. Shipping labels bear standard hazard statements since the piperazine core can carry mild irritation risk for eyes and skin. Packaging follows international transport codes, with bottles often capped tight and surrounded by desiccant packs to head off moisture uptake, preserving shelf stability over long hauls.
Synthesis often starts with 1,2-benzothiazole as the core, reacting via nucleophilic substitution or coupling routes to attach the piperazine group. Popular pathways tap benzothiazol-3-yl halides as electrophiles, stirring with piperazine in polar solvents, sometimes looping in a base like potassium carbonate to mop up any acids formed. Reaction conditions rarely demand exotic gear or high pressures, just a mindful touch with temperature control and a reliable stirring setup. Purification leans on recrystallization or column chromatography, depending on scale. Once the core is linked, many chemists then explore further appendages—alkyl, aryl, or acyl groups—spinning out analogs for biological or material screens.
The piperazine nitrogen atoms swing open doors to a basket of chemical reactions: acylation, alkylation, or sulfonation all prove straightforward, each shifting physical properties or biological activities in various directions. The benzothiazole ring has its own sites for subtle tweaks—halogen substitution, nitro group introduction, or exploration at the 6- and 7-positions. Medicinal chemists regularly attach different aryl or heteroaryl moieties aimed at tuning selectivity, solubility, or metabolic fate. Practice shows each change ripples through the molecule, sometimes boosting potency, sometimes knocking it sideways, but always teaching more about structure-activity relationships.
This compound appears in catalogs and literature under a handful of synonyms, each reflecting a different chemical naming tradition. 3-(1-Piperazinyl)-1,2-benzothiazole, Benzothiazole-3-piperazine, and BTZ-piperazine keep cropping up across European and Asian vendors. In pharmacology and patent filings, the names usually stretch to IUPAC length, yet researchers keep it practical, sticking to abbreviations and shorthand. Recognizing alternate names prevents confusion during collaboration and keeps procurement running smooth, especially in multi-site research projects or international drug development work.
Chemists working with 3-(piperazin-1-yl)-1,2-benzothiazole follow standard protocols: gloves, goggles, lab coats, and fume hoods form the basic gear. Even though acute toxicity lands in the moderate range for most analogs, skin exposure carries a risk of mild irritation or sensitization, so splash management sits front and center. Spills clean up easily with inert absorbents—no reactive vapor risks at the bench scale. Labs keep showers, eye-wash stations, and MSDS docs at arm’s reach, following institutional audits and global chemical safety guidelines. Disposal usually routes through organic waste streams, with pre-treatment if halogenated byproducts come up in custom syntheses.
Most scientists first encounter this scaffold in psychopharmacology, where it has powered generations of exploratory drugs chasing dopamine and serotonin activity. Some lines of research circle around its role in schistosomiasis and leishmaniasis treatment, particularly in regions hit hard by drug resistance. Outside medicine, manufacturers tap into the benzothiazole character for optical brighteners, molecular sensors, and corrosion inhibitors, especially where selectivity for transition metals pays off. Any effort to modify the piperazine allows technologists to tailor products for electronics, pigment chemistry, and specialty coatings—a sign of how far this molecule travels from its pharmaceutical roots.
Over the last decade, R&D teams have doubled down on rational design, drawing on 3-(piperazin-1-yl)-1,2-benzothiazole as a foundational motif. Screenings now reach far into computational chemistry, docking it with enzymes and receptors spanning neurobiology to oncology. Custom analogs get tested not only in cellular assays but in animal models, helping to build PK-PD data with greater nuance. Close industry-academia ties move leads rapidly from bench to pilot plant, with researchers publishing crystal structures, metabolic pathways, and in vivo activities to keep the knowledge cycle spinning. Expansion into nanotechnology and targeted drug delivery keeps opening new fields, with functionalized benzothiazoles leading development of smart diagnostics and nanomaterials.
Toxicologists continue to tease apart the health impact of exposure to benzothiazole derivatives. Most acute studies report mild to moderate toxicity, often relying on rodent models for early warnings. The piperazine portion brings its own suite of metabolic transformations, with phase I and phase II biotransformations leading to a range of metabolites, some less well-studied than others. Chronic exposure research stays ongoing, especially as materials science opens non-pharma routes to environmental release. Regulatory agencies track analytical standards, pushing for deeper understanding of degradation and safe disposal, cementing this compound’s reputation as both valuable and deserving of careful stewardship.
Researchers keep finding new inspiration in this molecule. With the growth of artificial intelligence and ML-driven drug discovery, screenings keep ramping up pace, matching this core with ever more diverse chemical libraries. Interest keeps growing in hybrid materials, turning benzothiazole-piperazine backbones into sensors that change color in response to biochemical targets or environmental stimuli. As personalized medicine carves a bigger share of treatment strategies, tailored derivatives aimed at rare disorders and specific patient profiles keep coming into view. Further, green chemistry keeps shaping synthetic routes and waste controls, driving chemical manufacturers to knock down energy usage and improve atom economy, all while keeping this distinctive scaffold at the front of innovation.
Working in a pharmacy, I’ve seen trends come and go, but certain chemical building blocks seem to stick around because they deliver real results. 3-(Piperazin-1-Yl)-1,2-Benzothiazole stands out for its strong presence in drug research, particularly in medicines that target the central nervous system. This compound belongs to a group of molecules that chemists use as starting points when designing drugs for mental health.
Medicines that help balance brain chemistry often contain complex ring structures, and this molecule fits right into that space. Drug developers have looked to 3-(Piperazin-1-Yl)-1,2-Benzothiazole as a base for antipsychotic drugs. The research shows that derivatives of this structure have played a major part in the design of atypical antipsychotics, which treat illnesses like schizophrenia and bipolar disorder. Just looking at the history of medications featuring a benzothiazole backbone, it’s clear this chemistry anchors real breakthroughs for people with serious mental health conditions.
What sets this compound apart is the way its structure interacts with neurotransmitter receptors in the brain. In my experience reading clinical papers and talking with researchers, I’ve found that these interactions matter because they mean the difference between broadside effects and targeted relief. Neurotransmitters like dopamine and serotonin are responsible for messages in the brain—and when those signals get out of balance, symptoms of psychosis, depression, or anxiety can set in.
3-(Piperazin-1-Yl)-1,2-Benzothiazole connects with both dopamine and serotonin receptors, which gives chemists a dual-action tool to address multiple pathways at once. This approach helps cut down on some of the worst side effects older medicines brought with them. Studies published in top medical journals, such as The Journal of Medicinal Chemistry, show this class of molecules keeps getting refined because it keeps working. That’s why pharmaceutical companies still invest in analogs based off this core structure, hoping to further improve outcomes while avoiding dangerous side effects.
All medicines carry risks, and the challenge with this family of compounds comes down to minimizing those risks for real patients. Fatigue, metabolic problems, and movement disorders remain concerns for people using central nervous system medications, despite improvements. As a pharmacy worker, I’ve seen the frustration up close—medications can help, but side effects can chase patients away from needed treatment. One way to move forward is with better screening for new derivatives at the early stages of development. By focusing intense attention on not just the direct effects, but also the late-arising and subtle ones, drug hunters can create safer options.
Beyond the lab, mental health services need to focus on the complete picture. Medical teams—doctors, pharmacists, therapists—must listen closely to patients who report side effects, and not brush off their experiences as minor tradeoffs. Partnerships between pharmaceutical developers and care teams could speed up feedback, helping scientists redesign trouble spots faster. There’s also value in focusing on patient education so that people understand both the benefits and the limitations of these medications. With ongoing support, breakthroughs anchored by molecules like 3-(Piperazin-1-Yl)-1,2-Benzothiazole can help people better manage their health and daily lives.
3-(Piperazin-1-Yl)-1,2-Benzothiazole holds the chemical formula C11H13N3S. Each molecule contains eleven carbon atoms, thirteen hydrogens, three nitrogens, and a single sulfur. Its molecular weight comes out to approximately 219.31 grams per mole. That number isn’t just trivia—it gives scientists and manufacturers the precision needed for safe experiments, careful mixing, and predictable therapeutic effects.
In any chemical lab, those numbers make or break the outcome. Dosing drugs gets tricky without a firm grasp of molecular weight. I remember chemistry coursework where the difference between 215 and 219 might mean throwing off the final concentration in a solution or miscalculating a reaction yield. Pharmacies double-check weights to avoid dosing errors. For scientists working with new benzothiazole compounds, that molecular weight anchors every calculation, mixing batch, and assay.
This compound doesn’t just bring numbers. It carries a unique backbone—a benzothiazole ring linked to a piperazine group. The benzothiazole core carries both nitrogen and sulfur, which gives the molecule a dual personality: aromatic and heterocyclic. Piperazine’s presence adds flexibility and allows tweaks to how the molecule interacts with receptors. Mix these traits and you wind up with a compound that’s been explored for everything from antipsychotics to anti-infectives. The fact that well-known medicines like ziprasidone also include similar scaffolds highlights the value of this backbone.
Drug development often pivots on tiny changes, like swapping a functional group or tweaking a ring structure. For chemists, the formula isn’t just paperwork. It’s the difference between targeting the right receptor and missing the mark. Efforts to stop antibiotic resistance, for example, look to chemical diversity in backbones like benzothiazoles. Knowing the formula lets researchers build libraries of subtle derivatives. It speeds up screening for new antibiotics or psychiatric drugs.
Beyond pharma, I’ve watched environmental scientists test fungicides and dyes that use similar scaffolds. Tight knowledge of molecular details leads to safer environmental handling and better detection in toxicity screens. Government safety data sheets demand specifics, and the weight factors into everything from safe storage to weighing out the right dose for animal studies.
One concern in labs comes from chemical confusion. Mislabeling or misreading a formula slows research and can ruin batches. Every synthetic route gets mapped out with the formula front and center; it sits at the core of the reaction plan. Chasing high purity starts with clear structure: a missing hydrogen throws things off, and adulterants get spotted by analysts who know every peak in a mass spectrum.
Better education, clear chemical labeling, and regular cross-checking with sources like PubChem lower risks. Open-access databases share details freely, which steers researchers toward quality. Collaboration across disciplines—chemists, clinicians, data analysts—pulls out insights faster and lowers chances for mistakes.
Having solid, specific data about chemicals like 3-(Piperazin-1-Yl)-1,2-Benzothiazole builds trust. From personal experience, I’ve seen new graduate students develop keen skill by cross-referencing, recalculating, and questioning every number in front of them. That habit carries over into industry and clinical trials, shaping outcomes for real patients. Accurate information on molecular structure and weight isn’t just academic—it’s the foundation of responsible, effective science.
Working with any research chemical comes with a set of rules that protect the people involved and safeguard long-term integrity. 3-(Piperazin-1-Yl)-1,2-Benzothiazole draws attention because of its use in labs focused on drug discovery and chemistry research. Skipping past textbook descriptions, one point sticks: mismanaging its storage can create all sorts of headaches, from wasted money to health hazards that never seem worth it. Having spent time in both university and commercial labs, I’ve learned that best practices are rarely one-size-fits-all, but some principles hold up under scrutiny every time.
Many chemists I know don’t take temperature advice lightly. For this compound, refrigeration often works best; storing it at 2–8°C avoids the decomposition you might see if a bottle sits on a warm shelf. Room temperature swings, sunlight, and constant movement make chemical stability a gamble. Each time an experiment depends on the original composition of a reagent, temperature control matters for success. I’ve seen researchers lose valuable samples to simple heat exposure—the ruin travels beyond the single bottle, affecting data reliability and budgets.
There’s nothing fancy here—dark brown glass vials help fight off light, and tight lids keep water out. Even if the substance looks fine right after delivery, ongoing exposure can push its structure to shift, slowly at first, then quickly when it’s too late to fix. Solid desiccants work wonders in storage cabinets, drying out air that would otherwise cause clumping or drive slow reactions. Following this careful routine, chemists can open up a bottle weeks or months later and trust in the material’s potency.
Every seasoned lab worker reads storage compatibility charts more like a survival guide than a chore. Acids, bases, oxidizers—each belongs in its lane. 3-(Piperazin-1-Yl)-1,2-Benzothiazole prefers neighbors that don’t set off chemical fireworks. I remember the ripple effect one bottle of oxidizer caused after sharing a shelf in grad school. We still talk about that learning moment. Cross-contamination, even between capped bottles, takes a toll on safety and experiment outcome. Labels should be clear, storage locations mapped, and everyone in the workspace should follow the same logic without shortcuts.
People shift jobs; notes get lost. I’ve seen lab logs act as lifelines, tracking who stored what, where, and when. Scanning a barcode or flipping a page saves hours of guesswork and debates during audits. Good records give each person accountability. If a problem arises, tracking down the storage details means resolution happens faster, without relying on memory or rumors.
No matter how advanced the facility, strong habits make the biggest difference. Regular training pulls new staff up to speed and reminds veterans not to skip steps. Physical locks, alarms, and temperature monitors provide extra assurance. Annual or even monthly audits highlight issues before they grow. From my experience, every lab team that invests in these checks cuts waste and shrinks risk. Improving storage is less about fancy gear and more about respect for the work and the people handling these materials.
Smart storage for 3-(Piperazin-1-Yl)-1,2-Benzothiazole comes down to a mix of discipline, the right tools, and honest respect for chemistry’s quirks. Simple routines keep chemicals viable, staff safe, and discoveries moving forward.
Working in laboratories and industrial settings, I’ve seen how chemistry brings both progress and risk. 3-(Piperazin-1-Yl)-1,2-Benzothiazole isn’t a household name, but it finds a place in research and pharmaceutical studies. Labs use it as a building block for more complex molecules, especially in neurological and antimicrobial drug research. The safety data for this compound deserves attention, not just in academic discussions, but among the people handling it.
This molecule carries some of the same risks as many benzothiazole derivatives. Direct contact can cause skin and eye irritation. Accidental inhalation might damage the respiratory system, bringing cough, dizziness, or shortness of breath. Swallowing this substance can cause stomach pain, nausea, or worse. Most handling guides recommend gloves, eye protection, and well-ventilated spaces even for routine work.
Safety data sheets (SDS) from top manufacturers list mild to moderate toxicity by common exposure routes. Chronic exposure risks remain under-studied, but benzothiazoles have made their way into industrial waste and sometimes pop up as environmental contaminants. The US Environmental Protection Agency and the European Chemicals Agency both highlight gaps in chronic toxicity research for many related compounds. For those working with them every day, this means a certain amount of precaution isn’t optional.
In my own time spent as a research chemist, I noticed many in the lab underestimate unknowns until something goes wrong. A careless spill or a split-second misstep with a pipette lands someone in the nurse’s office, or worse, in the hospital. It’s often the lesser-known intermediates like this one—not the infamous toxins—that end up causing injuries. The shift from ‘standard chemical’ to ‘hazardous substance’ requires only an accidental exposure.
For companies, overlooking this sort of risk damages both worker trust and public reputation. Workers who have suffered from chemical burns or acute symptoms due to overlooked protocols don’t forget the experience.
Health and safety laws around chemical handling have tightened over the years because enough people decided ‘good enough’ handling led to too many accidents. Regulations from OSHA, REACH, and local agencies each call for risk assessments, not just basic gloves and goggles. They expect clear labelling and up-to-date safety training. It’s not just compliance—it’s a way to keep teams healthy and projects on track.
A lot of emergencies get avoided not through fancy gear, but through good habits: labeling every container, reading the SDS before starting new experiments, and keeping the workspace uncluttered. Chemists who build these routines rarely face serious trouble.
Institutions can support their staff with proper ventilation, splash-resistant lab coats, and regular safety briefings. Workers benefit from dose-response data on even ‘niche’ chemicals like 3-(Piperazin-1-Yl)-1,2-Benzothiazole. Reporting and learning from small mistakes helps prevent bigger disasters down the line. In my experience, sharing real-life stories about potential accidents in weekly meetings keeps people alert more than posters or emails ever do.
Advances in chemical tracking and digital SDS access make it easier than ever to stay informed. Even veteran chemists can sometimes miss a new risk or an updated toxicity report. Easy access to information and clear company policy make a world of difference.
Chemicals like this won’t disappear from research and manufacturing. Treating them with qualified respect—and practical safeguards—prevents injuries, protects the environment, and sustains innovation.
3-(Piperazin-1-yl)-1,2-benzothiazole sits in a family of chemical compounds that pop up across several research domains. Labs and pharmaceutical developers prize this molecule for its range of biological applications. Still, the real difference often comes down to the grade and purity. Sitting at a bench, I’ve seen projects fly or flop based on these details. Anything used for drug development or biology gets extra scrutiny, because impurities, even trace ones, can muddy experiment results or raise safety flags.
Most suppliers push this compound with purity above 97%—usually, one can find offers for 98% or even 99%. HPLC takes the gold standard for verification. Shadowing an analytical chemist a few years ago, I learned just how carefully each chromatogram gets dissected before sending out a purity certificate. These numbers are not just sales hype. For pharma, even micro-level contamination can trip up synthesis, slow down screening, or make it through animal studies only to break down in clinical work.
Two main types pop up: research grade and pharmaceutical grade. Most of the time, labs pick research grade, which fits general R&D or screening work. This grade covers the basics and keeps prices reasonable. Sometimes suppliers toss around "analytical grade"—usually signaling an even tighter limit on unidentified peaks and metal content, but unless you’re running very sensitive assays, the slightly lower research spec does the job.
Then comes pharmaceutical grade. Here, quality control tightens up. Every bit, from water content to residual solvents, meets standards published in pharmacopeias. Patience and money buy this peace of mind. In drug development, regulatory agencies want to see tight documentation before greenlighting anything downstream.
I’ve walked through projects looking to pull SAR data where even a 1% impurity caused headaches in data interpretation. The wrong impurity can cover up or mimic activity, leaving teams chasing shadows. Across biotech, high purity pays for itself, avoiding reruns and last-minute surprises before publication or filing. More times than I’d like to admit, lower grade material created noise that wasted weeks.
Suppliers offer choices because budgets, risk tolerance, and downstream applications all pull in different directions. Cheaper, lower-purity batches still see use in early-stage work, especially if the group only wants to check feasibility. Yet, for anything touching animal models or preclinical steps, high-purity lots make the cut.
Quality certificates and certificates of analysis (COAs) give more than just numbers. They outline moisture content, melting point, and, crucially, show HPLC traces. Responsible suppliers often stand out by willingly answering follow-up questions about lot testing, recent analytical methods, and option for custom purities. In my experience, skipping these steps or leaving them to chance nearly always walks back into trouble later.
Choosing the right purity and grade often boils down to matching project needs and knowing what risks you can afford. If the plan is basic screening, a research grade above 97% may hit the mark. For anything on the path to the clinic, tightening the reins and demanding a full COA at 99% purity saves trouble later. Consult a chemist or regulatory lead—many headaches shrink under a few minutes of shared experience and a sharp look at the paperwork.
Meeting project goals safely, efficiently, and without wasted effort starts with good sourcing. Trustworthy suppliers, clear documentation, and matching the grade to the actual purpose lets teams focus energy where it counts.
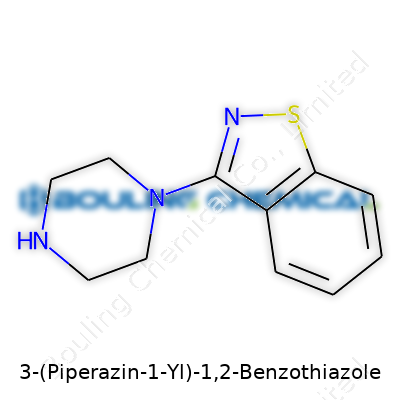
| Names | |
| Preferred IUPAC name | 3-(Piperazin-1-yl)-1,2-benzothiazole |
| Other names |
1-Benzothiazol-3-ylpiperazine 3-Piperazin-1-ylbenzo[d]thiazole 1,2-Benzothiazol-3-ylpiperazine |
| Pronunciation | /θriː paɪˈpɛrəˌziːn wʌn aɪl wʌn tuː bɛnˈzəʊˌθaɪəˌzoʊl/ |
| Identifiers | |
| CAS Number | 36556-72-2 |
| Beilstein Reference | 84134 |
| ChEBI | CHEBI:91483 |
| ChEMBL | CHEMBL138222 |
| ChemSpider | 22434552 |
| DrugBank | DB04220 |
| ECHA InfoCard | 03b55761-d8d7-4ffe-b4e4-8132caee6797 |
| EC Number | EC 620-771-4 |
| Gmelin Reference | 121344 |
| KEGG | C14155 |
| MeSH | D000070381 |
| PubChem CID | 187008 |
| RTECS number | JN9800000 |
| UNII | E5O9D12W9R |
| UN number | This product does not have a designated UN number. |
| CompTox Dashboard (EPA) | DTXSID4041343 |
| Properties | |
| Chemical formula | C11H13N3S |
| Molar mass | 263.37 g/mol |
| Appearance | White to off-white solid |
| Odor | Odorless |
| Density | 1.27 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.95 |
| Vapor pressure | 4.7E-7 mmHg at 25°C |
| Acidity (pKa) | 7.45 |
| Basicity (pKb) | 3.98 |
| Magnetic susceptibility (χ) | -72.62 x 10^-6 cm³/mol |
| Refractive index (nD) | 1.682 |
| Dipole moment | 4.52 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 185.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -2944 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | N05AX08 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause respiratory irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0-∞ |
| Flash point | 115.5 °C |
| LD50 (median dose) | Mouse oral LD50: 540 mg/kg |
| NIOSH | Not established |
| PEL (Permissible) | Not established |
| REL (Recommended) | 30 mg/day |
| Related compounds | |
| Related compounds |
1,2-Benzothiazole Piperazine Quinoline derivatives Benzothiazole derivatives 3-(Morpholin-4-yl)-1,2-benzothiazole 3-(Pyrrolidin-1-yl)-1,2-benzothiazole 3-(Piperidin-1-yl)-1,2-benzothiazole 3-Substituted-1,2-benzothiazoles |