The story of 3-Morpholinopropylamine traces back to the middle of the twentieth century, when demand for versatile amines surged across pharmaceutical and industrial sectors. Early documentation in chemical registries placed this compound among a wave of morpholine-based chemicals developed for novel reactivity and ease of modification. Researchers quickly noticed its amine functionality blended with morpholine’s stability, which opened doors for uses ranging from corrosion inhibition to epoxy curing. Steadily, labs integrated 3-Morpholinopropylamine into preparative organic chemistry, building upon earlier discoveries of morpholine as a solvent and intermediate. Over time, commercial synthesis processes streamlined its production, attracting interest from chemical plants and laboratories looking for efficiency without sacrificing purity.
3-Morpholinopropylamine presents itself as a clear, colorless to pale yellow liquid, sporting a faint amine odor one recognizes after some time spent in a chemistry lab. Often packaged in steel or HDPE drums, the chemical finds short and long-term storage viable so long as moisture and high temperature remain at bay. Its compatibility with organic solvents, along with its approachable toxicity profile compared with other industrial amines, sets it apart as a functional molecule in process chemistry. Bulk buyers span manufacturers of epoxy resins, pharmaceutical intermediates, and water treatment additives.
3-Morpholinopropylamine, with the molecular formula C7H16N2O and a molecular weight near 144.22 g/mol, brings some useful physical attributes to the chemist’s bench. Boiling occurs around 222°C, which makes it practical for reactions needing moderate heat. With a melting point below room temperature, users work with it as a stable liquid under normal conditions. Specific gravity sits near 0.99 at 20°C, hinting at easy miscibility with water and most organic liquids. The compound’s vapor pressure remains quite low, contributing to reduced volatility and diminished fire risk during handling. Chemical reactivity reflects the combined influences of a primary amine and a morpholine ring, allowing for versatile modifications.
Industry-standard 3-Morpholinopropylamine comes graded by purity, often exceeding 98%. Typical specifications include limits on water content (less than 0.5%) and the absence of related amines or other nitrogenous impurities. Labeling on containers features the UN identification number (common for all hazardous amines), batch identification, hazard pictograms, and recommended storage parameters. Regulatory documentation meets REACH in Europe and TSCA in the United States, reflecting a push for safe materials stewardship amid growing scrutiny over amine-based chemicals. In my experience, reading these labels closely saves a lot of trouble down the line since amine contamination can throw off sensitive downstream applications.
Most commercial preparation of 3-Morpholinopropylamine involves nucleophilic substitution using morpholine and 3-chloropropylamine hydrochloride under basic conditions. The reaction runs smoothly at elevated temperature and in the presence of phase-transfer catalysts, yielding the target amine after purification steps like distillation and extraction. Some manufacturing plants have shifted to greener catalysts or continuous flow reactors for better control and less waste. Research labs favor similar procedures because of reliability, and in both small and large-scale setups, reaction times and work-up protocols have been honed to minimize byproducts and streamline isolation.
As someone who has worked with 3-Morpholinopropylamine in a synthetic lab, its reactivity stems from the free primary amine group. It reacts rapidly with acyl chlorides to form amides, and with isocyanates to make ureas. The morpholine ring, though stable, can withstand a range of transformations, including alkylation or oxidation under mild conditions. Chemists often convert this amine into custom derivatives that retain some of that unique balance between flexibility and rigidity, which chemical engineers appreciate for process design. Among colleagues, we found its structure worthwhile in creating custom ligands for metal catalysis, and in personal experience, it opens up creative avenues for downstream synthesis in drug discovery.
Beyond 3-Morpholinopropylamine, catalogues and industry documents refer to it as N-(3-Aminopropyl)morpholine or 1-(3-Aminopropyl)morpholine. Some manufacturers abbreviate it to MOPA or use trade names embedding the morpholine or propyl component. Staying alert to these synonyms ensures correct ordering and avoids supply chain errors, an issue I have seen cause production halts more than once in formulation settings. CAS number 5037-47-6 provides an unambiguous identifier when other names fail to match up across borders or customs checkpoints.
Handling 3-Morpholinopropylamine safely means wearing chemical-resistant gloves, goggles, and lab coats because its amine functionality can irritate the skin and eyes. Spills produce pungent fumes, so an efficient fume hood is non-negotiable. Direct inhalation is unsafe, and accidental contact with strong oxidizers or acids risks exothermic reactions. Material safety data calls for dry, cool storage, away from incompatible substances such as chlorinating agents. Facilities handling multi-ton quantities use secondary containment and spill response protocols, as regulatory authorities expect these systems in place under OSHA or similar workplace safety standards. First responders and plant workers both benefit from up-to-date hazard training before engaging with shipments or conducting maintenance.
In industrial circles, 3-Morpholinopropylamine is best known for its role as a crosslinker in epoxy cure systems, where it enhances flexibility and impact resistance. Water treatment facilities turn to it as a neutralizing agent and corrosion inhibitor, often choosing this molecule for its low odor and reliable performance against scaling. Paint and coatings manufacturers add it to specialty formulations for enhanced adhesion on metal surfaces. Pharmaceutical research uses its structure to build new molecules with improved pharmacokinetics, while agricultural chemistry adapts its amine properties in the synthesis of certain fungicides and growth regulators. It even sees use in dye and rubber processing, making it an all-purpose chemical that crosses typical boundaries between sectors.
The pace of R&D involving 3-Morpholinopropylamine keeps accelerating across multiple disciplines. Current interests include tweaking its chemical backbone to extend its shelf life or integrating it into biocompatible polymers for wearable technologies. Teams at academic institutions are experimenting with its derivatives as secondary amines in medicinal chemistry, chasing new enzyme-inhibiting activity and better blood-brain barrier penetration. Process engineering groups at chemical companies are exploring microreactor synthesis paths, aiming to reduce energy consumption and limit environmental waste. Biotech startups seek to couple this amine with biomolecules for easier therapeutic delivery, while materials science pushes for improved resins that balance mechanical strength with environmental resistance.
Safety testing teams have scrutinized acute and chronic toxicity of 3-Morpholinopropylamine through inhalation, ingestion, and skin exposure studies. It causes moderate eye and skin irritation, and high levels in the air induce respiratory distress in animal models. So far, mutagenicity and carcinogenicity remain low or inconclusive according to published datasets, though long-term studies still emerge from regulatory science journals. In industrial use, limits on airborne concentrations keep workplace exposure within safe ranges. As regulations evolve, toxicity research has taken on proactive urgency, with public disclosure of findings and periodic updates for occupational safety officers.
Changes in global supply chains and consumer demand for greener chemicals will shape the path for 3-Morpholinopropylamine. Producers are investing in cleaner catalysts and more energy-efficient synthesis, a move that could reduce cost and shrink environmental footprints. Efforts to extend the chemical’s performance envelope—such as combining it with smart polymers or embedding it in hybrid nanomaterials—hint at new possibilities in engineering and medicine. Meanwhile, regulatory shifts on amine emissions and possible endocrine effects keep researchers refining toxicity profiles and downstream waste management practices. From ongoing dialogue between manufacturers, end-users, and scientific labs will come new guidelines and products that lean on the strengths 3-Morpholinopropylamine already brings to the market—reactivity, dependability, and adaptability.
I’ve spent a solid stretch dealing with the world of chemicals—either writing about them, working in labs, or helping businesses find out what’s useful, what’s just hype, and what’s downright risky. 3-Morpholinopropylamine sounds technical but plays an important part in some surprising corners of everyday life. Rolling out complicated chemical names might create distance, but understanding their place in regular products bridges that gap. 3-Morpholinopropylamine doesn’t grab headlines, yet it silently supports both industry and consumer life.
I’ve seen specialty chemicals come and go, but few stick around unless they offer lasting utility. 3-Morpholinopropylamine certainly finds a solid foothold as an ingredient in epoxy curing agents. Epoxy resin floors and adhesives in garages or hospitals rely on a hardener to produce their toughness and durability. That’s where this chemical steps in, offering improved drying times and a strong, lasting bond between surfaces. Construction workers, mechanics, and even home DIY enthusiasts may use products indirectly powered by this amine, even if the drum label mentions only a trade name.
Working with specialty coatings or paints, I came across formulas that leaned on 3-Morpholinopropylamine for specific properties. Paint producers crave chemicals that boost resistance against moisture and harsh cleaning chemicals. The amine structure brings these properties into paints and coatings, helping surfaces withstand more wear and tear.
My time consulting for industrial cleaning businesses introduced me to compounds built for tougher jobs than ordinary soap. Some industrial detergents contain 3-Morpholinopropylamine for its capacity to break down oily residues or greasy build-up on large metal parts. If you work in a factory or repair shop, the clean engine parts owe some thanks to this chemical.
Farm supply buyers might not know it by name, but certain crop protection products use 3-Morpholinopropylamine. Crop scientists turn to amines when designing pesticides and herbicides, ensuring that mixtures coat plant leaves evenly—and stick around in rain or wind. This is a balancing act: effective weed or pest control while keeping environmental risks in check. That’s a real concern for environmental advocates, and it’s why regulatory review matters.
Chemicals like this one demand respect for safety rules. Direct contact can irritate eyes and skin, and inhaling vapors may pose other health hazards. When I worked in the lab, gloves and goggles weren’t optional. The CDC and industry safety groups publish clear guidelines—nobody should ignore them. Responsible disposal also protects water sources and soil from contamination. Transparency, traceability, and proactive communication go a long way toward building trust in both industrial and agricultural use.
Wider use puts pressure on companies to evaluate supply chains. Concerns about workplace health, environmental spills, and the journey to greener chemistry have grown fast over the last decade. Producers—especially those shipping overseas—face stronger scrutiny. Developing safer alternatives, finding methods for better containment, and supporting employee training all count toward responsible stewardship.
My experience tells me that strong partnerships between manufacturers, safety regulators, and farmers drive practical change. Each group brings concerns and ideas to the table. Shifting to safer production, recycling containers, and regularly updating employee training materials all help reduce risk while maintaining productivity. Staying prepared for evolving regulations keeps products on the market and out of trouble. Whether you’re in industry or agriculture, paying attention to these details leads to a safer and more sustainable future.
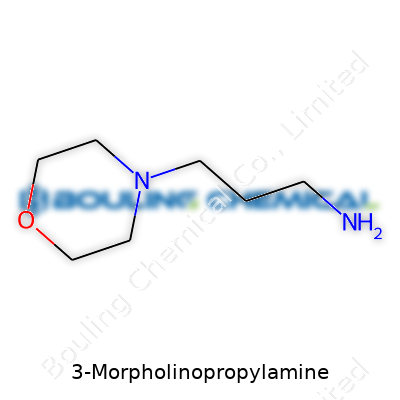
3-Morpholinopropylamine sits among the many chemicals making waves in both small-scale and industrial labs. Its chemical formula, C7H16N2O, spells out the basics—carbon, hydrogen, nitrogen, and oxygen combine to create a building block for other molecules. For anyone used to working behind a lab bench, the structure behind that formula matters as much as the letters and numbers themselves. This molecule starts with a morpholine ring—a six-membered structure carrying oxygen and nitrogen—and then links up with a propylamine chain. Chemists spot the formula and know it brings versatility for organic synthesis, specialty coatings, and emerging biotech.
Every chemist’s toolbox gets better with reliable molecules. I remember running reactions where small differences in structure turned a failed experiment into a publishable result. With 3-Morpholinopropylamine, its unique setup throws open options for linkages because that amine end group connects strongly to acids or other electrophiles, while the ring lends stability. In real terms, this means greater control when adjusting for temperature, solvent, or even the speed of a reaction. Structure always drives behavior—this is a lesson driven home by hands-on lab work far more than textbooks ever convey.
Many researchers use 3-Morpholinopropylamine as a curing agent for epoxy resins. Its chemistry brings flexibility to adhesives and coatings, tools relied on in aerospace or automotive projects. This compound also pops up in drug discovery and agrochemical prototypes, where shape and charge matter. Each time I faced a new application, digging into the structure and double-checking material data sheets proved wise. Like most amines, direct exposure can provoke skin or eye irritation, making gloves and goggles a must. Good habits don’t just tick boxes—they shape careers free of avoidable accidents.
Reading a label or formula only gets you so far. Building trust means sharing what experience teaches—how handling feels, what risks hide in plain sight, and where mistakes crop up. Peer-reviewed data backs up what you see on paper. The American Chemical Society put out case studies showing how 3-Morpholinopropylamine’s performance in curing agents can outshine traditional materials for impact resistance. Real-world testing keeps theory honest.
More students and small-scale labs want clearer safety data and honest talk about greener alternatives. Companies and educators could invest in better training and easier-to-read hazard labels, not just dense technical jargon. Stepping up communication between users, manufacturers, and regulators would shrink the gap between lab safety plans and daily routines. The basics—clear formulas, updated best practices, and willingness to learn—offer a path toward safer, smarter chemistry. Every honest conversation, every data-backed claim, builds a stronger bridge between discovery and practical use.
3-Morpholinopropylamine isn’t just a mouthful—it’s a useful ingredient in making coatings, resins, and pharmaceuticals. But few outside the chemical industry talk about what happens if you spill it on your skin, breathe its vapors, or let a bit splash near your eyes. This amine smells a little like ammonia, tingles on the skin, and burns in the nose. Plenty of folks figure it can’t be that bad. I’ve learned firsthand that even a small exposure can lead to headaches, skin redness, and a lingering chemical taste in your mouth. There’s nothing macho about risking your health for a shortcut.
Gloves always come out first on my bench. Nitrile or neoprene gloves hold up better to 3-Morpholinopropylamine than the cheap latex kind. I’ve seen coworkers think a cotton lab coat and thin gloves give enough protection. After a morning with a careless splash, they ended up red and itchy. Face shields and chemical goggles top the list too, not just basic glasses. A drop in the eye sends you to flush for 15 minutes, which feels much longer than you’d expect. Strong ventilation or a working chemical fume hood helps, because the vapors will drift into your breathing zone without you realizing. An NIOSH-approved respirator makes sense in small, less ventilated spaces.
I always check and label every container. Even a seasoned chemist can mistake a clear liquid for water or alcohol. In my early days, an unlabeled flask containing 3-Morpholinopropylamine sent a lab partner scrambling to the eyewash station. That’s not the memory you want on your resume. Spills on bench tops should get a solid wipe-up with absorbent pads made for chemicals, followed by a proper decontamination wash instead of a quick swipe with a paper towel.
Some chemicals quickly break down or dilute in water, but 3-Morpholinopropylamine can react with oxidizers, acids, and other substances. Dumping it down the drain—for the sake of convenience—runs the risk of unexpected reactions in your own pipes or in the wastewater system. Waste goes into clearly marked containers for disposal by professionals, never a regular trash bin. It’s easy to think “just this once,” but that’s usually the time the accident happens.
Every well-run site makes training part of the routine. Even if you think you’ve heard it all, one missed step—or a new protocol—can make the difference between a clean record and a trip to the emergency room. Sharing tips from real slip-ups with younger techs isn’t about scaring them; it’s about building respect for the chemical and for each other. If someone looks unsure or skips PPE, I make a point to speak up. That small nudge often keeps trouble out of the lab.
Emergency showers and eye washes need a clear path. In practical terms, that means no stacking boxes or blocking routes. I check for fresh eyewash solution and working showers, not just the posted inspection tag. No one wants to use them, but everyone is grateful when they’re ready. Keeping spill kits stocked and knowing where to find them saves time if something ever does go wrong.
After years of handling 3-Morpholinopropylamine and similar chemicals, the lesson hits home: safety isn’t a one-time talk or a checklist. It’s a practice built on habit, listening to each other, and taking pride in sending everyone home healthy. Chemistry opens doors, but caution keeps those doors open for the long run.
3-Morpholinopropylamine, used in things like creating pharmaceuticals and specialty chemicals, comes with a list of safety issues if not stored right. Anyone who’s worked around amine-based chemicals knows the risks aren’t just theoretical. Health and safety slips can lead to big problems — not just in the lab, but right through the supply chain. A single lapse affects workers, the equipment, and the environment. So, making strong choices about how and where chemicals sit on the shelf matters.
Heat and humidity mess with 3-Morpholinopropylamine. Direct sunlight changes its makeup, making it much more dangerous if spilled or inhaled. I once saw bottles left out on a sunny windowsill during a university internship. Instead of staying clear, the liquid grew discolored and started to smell much sharper. That incident cost the department a lot — wasted product, corrosion on shelving, a few weeks of unnecessary worry.
Keep it in a cool, dry room, away from sources of ignition and out of direct sunlight. Temperatures above 25°C raise the risk of vapor buildup, which brings its own set of headaches. Ventilation saves a lot of trouble. Storage areas shouldn’t double as closets where equipment piles up against drums. Airflow clears out dangerous fumes before they go unnoticed.
Glass and high-density polyethylene containers don’t react the way mild steel or low-grade plastic do. Over time, amines eat away at poorly chosen bottles, creating leaks and contaminating what’s inside. Once, on a consulting job, a manufacturer tried to cut corners using mismatched containers for amines. The result looked like salt rings and sticky patches everywhere — and risked an expensive waste treatment cleanup.
Label every container. Even experienced hands mix things up, especially when handling dozens of nearly identical bottles. Standard safety labels that highlight chemical name, hazard class, and the date poured or received go a long way in preventing confusion. Use secondary containment trays. Small spills stay contained, and cleanup becomes quick and routine, rather than turning into an emergency.
Storing 3-Morpholinopropylamine away from acids cuts down on violent reactions like exothermic spills, toxic fumes, and pressure buildup. The chemical can react dangerously if acids, oxidizing agents, or even some cleaning products sit nearby. I remember a paint production plant that suffered an evacuation because someone stored a barrel of strong acid on the wrong rack. The mix let off fumes that set off alarms and brought production to a halt.
Safety data sheets back this up. They recommend isolation from incompatible materials, which includes strong oxidizers and food products. Cross-contamination doesn’t just endanger workers. It can ruin entire batches, costing thousands in lost production and cleanup.
Clear policies and regular trainings turn good storage practices into habit. Everyone, from trainees to chemists, should know exactly where chemicals live, how to report leaks or odd smells, and how to identify improper storage before it turns into a crisis. Inspections every few weeks help spot cracks, leaks, or evaporated products. These checks catch problems before inspections by local authorities do. Keeping logs tracks changes over time, making it easier to see which products go bad or need disposal.
Protecting people, product value, and the wider environment starts with how a chemical gets stored. It pays off to build those habits right from the start and reinforce them at every stage in the supply chain.
Most people outside the world of chemistry probably haven’t come across 3-Morpholinopropylamine in everyday life, but this compound quietly finds its way into a surprising number of products. It’s a clear, colorless liquid with a faint amine odor—a bit fishy and slightly sharp, typical for many amines. On the bench, watching someone handle a bottle of this liquid, it’s obvious why lab techs prefer gloves and goggles. Contact on skin brings out a quick sting, and the smell hangs in the air.
This amine clocks in with a density a bit below water, about 0.98 g/cm³ at room temperature. Pour some into a beaker and water floats on top. Density matters a lot during phase separations and workups, so knowing this simple fact saves time and prevents mess in the lab. The boiling point lands around 220°C, which means it stays liquid under most room and even industrial temperatures, and you need significant heat to distill or remove it.
Drop this chemical into water and it dissolves easily. It blends well with alcohol and other polar solvents, but in something like hexane, it barely mixes. Manufacturers take advantage of this property for formulating coatings, resins, and additives. From personal experience, cleaning glassware after working with this amine is much simpler than after using some oily, hydrophobic chemicals—plain water and soap usually do the trick.
3-Morpholinopropylamine pours with a low viscosity, feeling much thinner than oils but a bit thicker than water. It moves quickly in and out of pipettes. This matters when dosing precise amounts. Industrial users or researchers working with automated pumps can trust it won’t clog tubing or stick to surfaces.
This amine turns up clear, without any usual haze or suspended matter. Chemists keep an eye out for any cloudiness—it signals contamination or decomposition. In practice, color and clarity provide a real-world check on chemical quality, much like looking for clarity in cooking oil before use.
Businesses using 3-Morpholinopropylamine in large volumes stake a lot on these physical traits. A lower boiling point would create more vapor losses and workplace hazards; a higher density could impact mixing ratios; poor water solubility would limit applications. Factories and labs avoid surprises and accidents through a clear understanding of these everyday facts.
With a low flash point, this amine doesn’t pose a serious fire risk under standard conditions, but its skin-irritating nature reminds workers to respect proper safety measures. Sealed, labeled containers and well-ventilated storage matter as much as the compound’s boiling point or solubility. In my own former labs, strict inventory control and double-checking labels prevented confusion with similar-looking liquids.
Learning about the hands-on properties of chemicals like 3-Morpholinopropylamine builds better habits for safety and precision in any workplace. Training programs work best when they spotlight everyday handling tips—from avoiding skin contact to properly ventilating workspaces—based on firsthand knowledge of these core features.
| Names | |
| Preferred IUPAC name | 3-(Morpholin-4-yl)propan-1-amine |
| Other names |
N-(3-Aminopropyl)morpholine 3-(Morpholin-4-yl)propan-1-amine 3-Aminopropylmorpholine |
| Pronunciation | /θriː-mɔːrˌfəˈliːnəˌproʊpɪlˈæmɪn/ |
| Identifiers | |
| CAS Number | 5037-09-6 |
| 3D model (JSmol) | `3d-3819548` |
| Beilstein Reference | 1342104 |
| ChEBI | CHEBI:85231 |
| ChEMBL | CHEMBL15829 |
| ChemSpider | 14213 |
| DrugBank | DB08373 |
| ECHA InfoCard | ECHA InfoCard: 100.096.804 |
| EC Number | EC 203-841-9 |
| Gmelin Reference | Gmelin Reference: **83416** |
| KEGG | C06053 |
| MeSH | D017247 |
| PubChem CID | 12216 |
| RTECS number | TZ6793000 |
| UNII | MC642913E5 |
| UN number | UN2735 |
| CompTox Dashboard (EPA) | DTXSID6031877 |
| Properties | |
| Chemical formula | C7H18N2O |
| Molar mass | 130.20 g/mol |
| Appearance | Colorless to yellowish liquid |
| Odor | Ammonia-like |
| Density | 0.98 g/mL at 25 °C (lit.) |
| Solubility in water | Miscible |
| log P | -0.40 |
| Vapor pressure | 0.5 mmHg (20°C) |
| Acidity (pKa) | 9.40 |
| Basicity (pKb) | 5.55 |
| Magnetic susceptibility (χ) | -54.3×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.456 |
| Viscosity | 5.1 mPa·s |
| Dipole moment | 2.49 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 241.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -114.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4473.6 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes severe skin burns and eye damage. Toxic to aquatic life. |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "H302, H314, H412 |
| Precautionary statements | Precautionary statements: "P261, P280, P305+P351+P338, P310, P337+P313 |
| NFPA 704 (fire diamond) | 2-3-1 |
| Flash point | 75°C |
| Autoignition temperature | 199 °C |
| Explosive limits | The explosive limits of 3-Morpholinopropylamine are: "1.6% (LEL) - 12.5% (UEL) |
| Lethal dose or concentration | LD₅₀ oral rat 2,639 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 2,800 mg/kg |
| NIOSH | 85-73 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 3-Morpholinopropylamine: Not established |
| REL (Recommended) | 3-MPA |
| IDLH (Immediate danger) | IDLH: 100 ppm |
| Related compounds | |
| Related compounds |
Morpholine 4-(2-Aminoethyl)morpholine N-Methylmorpholine 3-Aminopropylamine Diethanolamine |