The journey of 3-Methyltetrahydrothiophene 1,1-dioxide tracks closely with advances in organosulfur chemistry. In the mid-20th century, laboratories buzzing with innovation started diving deep into sulfur-containing heterocycles, looking for novel building blocks. As industries searched for more functional intermediates—especially those resilient to oxidation or fit for tuning chemical reactivity—compounds like 3-Methyltetrahydrothiophene 1,1-dioxide stepped into the spotlight. Back in university days, I remember lectures on sulfolanes and related molecules, the professor flicking through early patents and synthesis notes that mapped out the first practical preparations of these dioxides. Chemists were eager to push boundaries not just for the sake of new molecules, but for robust solvents, pharmaceuticals, and specialty chemicals that could keep up with the post-war chemical boom. Industrial adoption followed once commercial-scale oxidation and purification processes got ironed out, opening the doors for research labs worldwide to incorporate this sulfur heterocycle into their own toolkits.
3-Methyltetrahydrothiophene 1,1-dioxide, also referred to as 3-methylsulfolane, fills a specific niche where a polar, aprotic medium is needed, or where a scaffolding for further molecular tinkering is in order. Its five-membered ring carries a sulfur atom doubly bonded to oxygen—making it a robust sulfone, not easily knocked down by ordinary bases or reducing agents. Across chemical supply catalogues, this compound usually ends up categorized under research chemicals or specialty solvents. Colleagues in both academia and the process industry talk about it when describing tough separation jobs, kinetic studies, or advanced synthesis strategies. The “dioxide” part signals oxidative modification of its sulfur, setting it apart from mere thioethers or sulfides and giving it a special place in the broad world of organosulfur chemistry.
The substance forms a clear, colorless to pale liquid, sometimes sold as a low-melting solid in chillier climates. Its melting point usually lands around 28°C, while the boiling point can surpass 280°C, which often translates to handy thermal stability during synthesis. Its density generally clocks near 1.26 grams per cubic centimeter, which I’ve found compares well with classic solvents like DMSO or sulfolane. Polarity plays a huge role in why chemists gravitate toward this compound. The strongly electron-withdrawing sulfone group boosts its ability to dissolve polar substrates or support sophisticated reactions. Chemically, it's stubborn—it shrugs off dilute acids and resists many bases. Yet, once you line up the right nucleophile or run conditions just warm enough, you can coax the methyl group or the ring system into taking part in substitution or ring-opening chemistry. The compound sports a modest vapor pressure, so it tends not to fill a lab with fumes.
Suppliers typically provide a purity level of at least 98 percent, often accompanied by a chromatographic fingerprint (GC or HPLC trace). They note water content, because trace moisture can skew reactions, especially in organometallic work. Packaging comes in amber glass bottles, since sulfone rings like this one can slowly degrade if exposed to sunlight or high humidity. Labels carry the chemical formula C5H10O2S, a CAS registry number for easy traceability, batch numbers, and hazard codes in line with GHS standards. Over the years, I’ve learned the hard way to always check these details—the odd mislabeled sample in a communal fridge can throw off days of planning. Inventory lists also cite storage requirements, flagging the need for a cool, dry place away from incompatible reagents.
Lab-scale routes usually start with 3-methyltetrahydrothiophene or its parent thietane. Oxidation forms the sulfone: hydrogen peroxide, sometimes paired with acetic acid, turns the sulfur atom into a sulfone group without wrecking the carbon skeleton. Alternative methods tap into m-chloroperoxybenzoic acid (mCPBA) or sodium periodate for selective oxidation. My own experiments proved that keeping the reaction cold initially helps dodge unwanted over-oxidation, while slow addition of oxidant yields a cleaner outcome. The workup typically involves extraction with nonpolar solvents, washing away the byproducts, and then distilling under reduced pressure for purity. Some chemists prefer a column purification step to really polish the sample, especially if analytical work or sensitive reactions are on the agenda.
This sulfone ring stands tough against typical reduction; you can really lean on it as a backbone during steps that would crumble sulfides or selenides. Specialists in synthetic organic chemistry appreciate its methyl group as a point of entry for Friedel-Crafts alkylation, halogenation, or functional group exchange. One friend, working in a medicinal lab, used 3-methyltetrahydrothiophene 1,1-dioxide to build a suite of intermediates for anti-inflammatory compounds—using the methyl group as an anchoring spot for further elaboration. Under strong conditions, the molecule takes part in nucleophilic substitutions, either at the methyl or sometimes at the ring carbons when faced with the right pairing of base and temperature. Ring opening with nucleophiles opens doors to linear sulfones or functionalized chains, useful both in small-scale synthesis and bulk ingredient production. The ring’s rigidity, offset by its polar environment, lets you tune the electronics in ways straight-chain sulfones can’t offer, giving more control over reaction rates or selectivity.
Besides its IUPAC name, commercial and research circles use several aliases. Chemistry catalogues often list it as 3-methylsulfolane or methyl sulfolane, sometimes with the “1,1-dioxide” tag attached for clarity. Older sources, or material safety data sheets from international suppliers, may reference it by derivatives or reserve the longer sulfolane nomenclature. During collaborative projects spanning multiple universities, I saw Russian, Japanese, and German teams translate the name to fit their own conventions. But in most project meetings, “methyl sulfolane” or “3-methylsulfolane” does the job—it’s direct, and lab communities pick it up fast.
Working with sulfone solvents and intermediates, I always prioritize protective gear—lab coats, gloves, and goggles, regardless of the compound’s relatively tame volatility. Exposure to the eyes or skin can cause mild irritation, so spill protocols matter. Regulatory data generally rate it as having low acute toxicity, but I’ve seen labs run ongoing monitoring, especially during scale-up work, to minimize both inhalation and long-term contact. Waste handling takes center stage too. Disposal involves sealed containers, slated for incineration or secure chemical waste processing. Local and international safety guidelines require well-ventilated workspaces, and process setups call for secondary containment to prevent cross-contamination. Fire risk is minimal, but emergency showers and eyewash stations stay close at hand. Focused training makes a huge difference—every new team member needs hands-on demos before handling a new class of organosulfur chemicals.
My old research group used 3-methyltetrahydrothiophene 1,1-dioxide mostly as a high-performance solvent, especially when polar aprotic conditions could tip a tough synthesis in our favor. It carves out a space where classic solvents fall short—high-boiling, polarity just right for roasting out difficult separations or running tricky kinetic studies. Process chemists at industrial plants sometimes lean on it for extraction work or to stabilize sensitive intermediates during batch processing. It anchors multi-step syntheses both in the pharmacy sector and specialty materials, occasionally serving as a starting framework for active pharmaceutical ingredients or polymer additives. In analytical labs, the compound brings out sharper separations in chromatography when mixed with other polar solvents. Recent trends show a slight uptick in its use for lithium battery electrolyte research, tapping the ring’s stability and sulfone’s electronic effects to improve performance under high-voltage cycling.
Ongoing R&D tracks the sulfone’s adaptability for building new pharmaceuticals, high-functioning materials, and as a benchmarking solvent for electrochemistry tests. The toolbox keeps growing—chemists explore selective halogenation and functionalization strategies, aiming for new routes to pharmaceutical building blocks. Green chemistry initiatives now steer preparation methods toward less hazardous oxidants or recyclable catalysts. The molecule’s ability to host modifications without losing stability makes it a valuable candidate for catalyst support structures, or as a backbone in designer ionic liquids. Recently, international teams mapped out biocatalytic pathways for selective oxidations, hoping to short-circuit step counts and reduce waste. Conferences highlight both academic and industrial uses, with speakers swapping ideas for maximizing selectivity or recycling outputs. Investment in advanced NMR and chromatography speeds up the work, making product identification and purity checks more reliable than ever.
Available toxicity data place 3-methyltetrahydrothiophene 1,1-dioxide in a middle ground—it doesn’t carry acute toxicity flags that some sulfur heterocycles have, but solid animal and cellular studies remain on the modest side. The ring’s polar sulfone group helps it clear from most biological systems faster than some of its less-oxidized cousins, but chronic exposure questions linger. Some industrial hygiene teams keep an eye on low-level vapor generation, even with its low volatility. Longer-term studies, especially those examining metabolic breakdown in rodents or aquatic species, push for updated MSDS sheets and smarter waste management. The research community sees value in building a more complete toxicological picture, both for worker safety and to clear hurdles in pharmaceutical or materials approval processes. Improved analytical methods now track trace residues in finished formulations or environmental samples, keeping regulators in the loop.
3-Methyltetrahydrothiophene 1,1-dioxide won’t displace the chemical giants like DMSO or sulfolane, but it brings enough unique features to stick around the specialty chemicals scene. Green chemistry momentum could push for milder, less resource-intensive oxidation strategies—electrochemical or enzymatic methods stand as strong candidates for the coming decades. Growers of advanced electronics and high-performance batteries want more stable, efficient solvents, and this sulfone’s profile matches some of their most urgent needs. Pharmaceutical innovators eye modifications of the ring for next-generation drug scaffolds or carrier molecules. Regulatory standards will keep evolving, and the community must keep up with safety, waste, and best practices to stay competitive. My work with interdisciplinary teams tells me that deep collaboration—across academia, industry, and regulatory bodies—will shape both the use and stewardship of 3-methyltetrahydrothiophene 1,1-dioxide for years to come.
Stepping into daily life, few folks realize how much chemistry shapes what we use and touch. Take 3-Methyltetrahydrothiophene 1,1-dioxide, for instance—a mouthful, but with real-world jobs you probably never consider. It’s not a common household product, and most never see it on labels, but its fingerprint is everywhere in the supply chain of things many rely on daily.
Pharmaceutical scientists often seek out reliable compounds that improve how drugs work. This molecule, better known to researchers as Sulfolane derivative, steps up there. Its ability to help dissolve other compounds turns it into a trusted solvent in drug synthesis runs. Without solvents like this, chemists face headaches getting actives to combine or purify, which limits the medicines making it from laboratory to pharmacy shelves. The cost of failed reactions and wasted resources climbs quickly if the wrong solvent is chosen; in my own years around research, I saw how the wrong pick could set a whole week’s progress back.
Factories use this compound for more than dealing with pills. Think of things like specialty plastics, crop protection chemicals, or permanent flame retardants: many start with raw materials that need gentle but tough solvents to coax the reactions. That’s where this molecule steps in again, making it possible to clean up the final products or move along tricky chemical reactions. Without stable and efficient solvents, manufacturing costs rise, and companies face tighter bottlenecks on volume.
Not every compound breaks down in safe or predictable ways. 3-Methyltetrahydrothiophene 1,1-dioxide stands out because it resists reacting with a lot of process chemicals, so unwanted byproducts don’t pile up. That makes disposal easier and worker exposure risks lower. In an era with tighter regulation, lower emissions, and strict workplace rules, companies can’t ignore the long-term savings and safer working environments this quality brings. Efficiency isn’t just about saving time and money; it’s about cutting health and environmental impact. I remember seeing a process switch to safer solvents and the huge relief during audits—fewer hazardous waste forms and fewer worried faces on the shop floor.
Whether working on new battery designs, polishing semiconductors, or mixing up novel dyes, reliable solvents matter. The industry wants chemicals that won’t run out soon or pollute the streams. 3-Methyltetrahydrothiophene 1,1-dioxide fits in as both stable and widely sourced. That gives it an advantage over rare or newly invented solvents, which sometimes prove too costly or untested at scale.
As more companies turn over old practices and start phasing out legacy chemicals, there’s reason to push for more research into the health impacts and recyclability of this compound. Suppliers and regulators should keep close watch on supply chains and workplace safety, asking for better documentation, more toxicology reports, and clearer training for staff who handle, transport, or dispose of these chemicals. Education and transparency, not shortcuts, set up workers and end users for the best outcome.
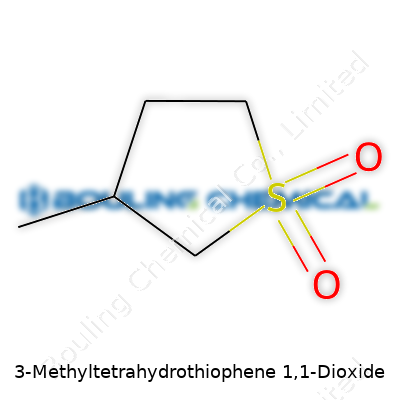
3-Methyltetrahydrothiophene 1,1-dioxide packs quite a punch for a small molecule. At a glance, the name looks intimidating, but in reality, this compound just puts a cool tweak on the basic sulfur heterocycle form. Imagine tetrahydrothiophene, a sulfur-containing five-membered ring, then add a methyl group at position three. Now, take that sulfur and turn it into a sulfone — that’s the “1,1-dioxide” part. Right away, you’re left with a molecule shaped kind of like a drawn-out pentagon, featuring one sulfur atom double-bonded to two oxygen atoms.
The chemical formula, C5H10O2S, gives a hint at its balanced simplicity. Carbon and hydrogen stay pretty predictable, but it’s the sulfur’s bond to oxygen that changes the chemical personality of this compound. That sulfone character brings a new set of properties — higher polarity, greater resistance to reduction, and a notable impact on how it dissolves in various solvents.
I’ve spent enough time around labs and process plants to see how a tiny change in structure can flip a material's usefulness. Here, adding that methyl group at the third carbon boosts volatility just enough to set this chemical apart from its close relatives, especially in gas detection and environmental tracking. The sulfone part dials down reactive “smelliness” typical of thioethers, which has to be an upgrade for any technician who spent too many hours sniffing out trace contaminants. The stability under harsh conditions also makes it reliable for controlled-release or specialty chemical applications where unpredictable breakdown just isn't an option.
Structurally, the molecule is easy to visualize for anyone with a basic chemistry background. Picture a sulfur atom sitting in the middle of the ring, double-bonded to two oxygen atoms, surrounded by a mostly-alkyl framework, and a methyl side group that refuses to go unnoticed. This arrangement not only shapes its reactivity but also how the body and the environment will handle it. It’s less prone to easy breakdown or transformation, which is both a blessing and a headache, depending on where it ends up.
Experience shows that overlooking a single oxygen or a lone methyl group can cost time, money, or safety. Regulatory teams in Europe and the US both flag this structure’s sulfone character as crucial. Extra oxygen atoms grab attention in environmental assessments — metabolites from sulfone-containing compounds linger longer and can behave quite differently compared to more basic sulfur rings.
When tackling waste disposal or long-term exposure, project leads benefit from knowing this structure inside and out. Newer modeling tools use the precise arrangement of those oxygen and sulfur atoms to predict long-term fate. Sometimes, increased polarity helps with easier cleanup, but at other times, sluggish breakdown throws a wrench in the works. Working with colleagues in remediation, I’ve watched teams choose different processes for a sulfone versus a sulfide, and that decision traces back directly to understanding structures like this one.
No one should overlook the small changes that a methyl group or an extra oxygen brings to a sulfur-containing ring. Handling something like 3-methyltetrahydrothiophene 1,1-dioxide without understanding the significance of its structure opens the door to wasted resources or unplanned regulatory hurdles. Industry players making solvents or chemical intermediates keep looking for tweaks — often, the right structural arrangement boosts efficiency, minimizes risk, and unlocks brand-new applications. Diving into the atomic-level details and seeing how these play out in day-to-day operations, research or end-use safety, always pays off in the long run.
Spend any time working with chemicals like 3-Methyltetrahydrothiophene 1,1-Dioxide and you learn pretty quickly: it pays to know what you’re dealing with. Unlike household cleaners or things most folks touch every day, this compound doesn’t usually show up outside industrial and research settings, so average people don’t have much reason to think about what it does to skin and lungs.
A major concern with handling organosulfur compounds boils down to volatility and toxicity. 3-Methyltetrahydrothiophene 1,1-Dioxide belongs to a class of chemicals sometimes used as solvents or intermediates. From the standpoint of acute toxicity, published data suggests this is no fluffy bunny compound. Direct exposure can irritate eyes, skin, and airways. Spills won’t eat straight through gloves or glass, but regular latex or nitrile gloves sometimes don’t last if there’s a splash or immersion, which shows why checking glove compatibility charts matters. A face shield does more than look impressive — it keeps accidental splashes from ruining your week.
Few people look at a bottle and see potential danger unless there’s a big skull-and-crossbones. But I've seen labels that barely hint at a chemical’s punch — poor hazard labeling or incomplete Safety Data Sheets turn risky compounds into a gamble. In the case of 3-Methyltetrahydrothiophene 1,1-Dioxide, its vapor is mild compared to solvents like benzene or toluene, but long-term inhalation can bring on headaches, dizziness, and in some cases, more serious issues with nerves or organs. Chronic low-level exposure in a poorly ventilated space builds up trouble, especially if managers downplay employees’ concerns or ignore proper safety measures to save a buck.
I’ve handled similar sulfur-based solvents in university labs where fume hoods and eye wash stations got as much routine use as pipettes. There’s a tendency in small companies to skip well-ventilated setups and simply “open a window.” The errors start small: skipping gloves “just for a second,” not storing bottles in a cool, dry place, running short on proper spill kits. These shortcuts line up perfectly for trouble.
Guidance from regulatory bodies like OSHA and the European Chemicals Agency underscores the need for control methods before anyone puts on a lab coat. Exposure limits exist for good reason; stories of burned skin, hospital trips, and ruined experiments didn’t start in a vacuum. Reliable risk assessment relies on both a top-down approach (clear policies, real training, enforced storage guidelines) and a bottom-up push from folks who actually handle the materials to speak out when short cuts creep in.
Investing in good storage cabinets, certified hoods, and requiring regular safety training for new staff can spell the difference between a productive lab and a dangerous one. I’ve seen labs try to get by with the bare minimum, acting only after an incident turns into paperwork and insurance claims. Prevention almost always costs less than cleaning up after a chemical accident.
Forget hoping for one-size-fits-all safety advice. Risk drops dramatically when companies give practical, experience-backed guidance tailored to their own operations. Routinely check material inventories. Pair new workers with those who’ve seen mistakes firsthand. Make spill reports a badge of responsibility, not a sign someone messed up. Keep up with updated studies on long-term exposure — as more data appears, risks can turn out higher or lower than old assumptions suggested.
3-Methyltetrahydrothiophene 1,1-Dioxide brings clear risks. Nobody benefits from half-measures or outdated data. With the right respect for its hazards — and investing in real-world experience, up-to-date protective equipment, and empowering employees to speak up — this compound can be handled effectively and safely.
As someone who’s spent years working around specialty chemicals, I’ve come to respect the power of simple routines. The importance of good storage for substances such as 3-Methyltetrahydrothiophene 1,1-dioxide goes far beyond regulatory fuss. This chemical, often used in organic synthesis and material sciences, demands more than a locked supply cabinet. Storing it well keeps everyone safe and ensures it does its job when needed most.
Every chemist learns to seal up bottles because moisture is often the silent thief in a lab. 3-Methyltetrahydrothiophene 1,1-dioxide reacts with water, which risks chemical breakdown or hazardous by-products. The fix? Use airtight containers with reliable seals. I’ve seen humidity wreck dozens of materials, and more than once, it led to headaches during audits and wasted hours on cleanup. Desiccators make a big difference. Silica gel or molecular sieves inside storage cabinets often save both time and money down the line.
Not every compound blows up when left near a heat source, but elevated temperatures are never a minor detail. Heat speeds up degradation, which means less purity and reliability. I’ve kept this chemical in a temperature-controlled area, usually away from sunlight, radiators, or hot labs. Room temperature is usually fine if the place remains stable and on the cooler side. Never stack containers near equipment that might generate heat – it isn’t just about fire risk, but slow decay that creeps up on you.
Fumes from sulfur-containing compounds can sneak up on you. 3-Methyltetrahydrothiophene 1,1-dioxide can give off odors and vapors that aren’t just unpleasant—they may be harmful if they build up. Over the years, I’ve learned the value of dedicated ventilated chemical storage cabinets. Good airflow prevents accumulation of any vapors. I’d never recommend using general office cabinets or wooden shelves because they trap smells and, over time, degrade the storage area. Investing in proper chemical cabinets pays back in clean air and peace of mind.
Many accidents involving lab chemicals come from poor segregation. Mixing incompatible substances, like oxidizers and reducers or acids and organic solvents, raises the risk of fire or toxic fumes. I store sulfur-containing compounds like this one away from strong acids, bases, and other reactive agents. Simple color coding or labels help team members know what belongs together and what doesn’t. This habit has saved me from more than one close call in shared labs.
Even a perfect storage setup fails if people aren’t ready to use it right. Regular walkthroughs and labeling updates keep everyone on the same page. I’ve never regretted walking a new team member through storage protocols. Even experienced professionals miss updates or forget product quirks—especially in stressful moments. Training is just as important as the right shelves and containers.
As automation and smart inventory systems improve, we keep seeing more ways to avoid mistakes. Sensors can now warn you if a cabinet gets too humid or hot. Digital checklists remind everyone to reseal containers and note expiration dates. These tools don’t replace experience, but they definitely support it. We need manufacturers, safety teams, and lab staff to talk often, so storage practices keep pace with research demands and new hazards. For now, strong habits, a watchful eye, and solid training remain the best insurance for storing 3-Methyltetrahydrothiophene 1,1-dioxide safely and reliably.
3-Methyltetrahydrothiophene 1,1-dioxide is not a material you find at hardware stores or the average chemical supplier. Chemists, researchers, and folks working in the realm of organic synthesis run into this compound most. Its role in selective oxidation reactions and pharmaceutical research stands out more than in everyday use. People involved in specialty chemical production or advanced material synthesis look for this stuff for a reason, not curiosity. That focus tends to keep it off the shelves of mainstream outlets and in the hands of those who have a clear professional purpose.
Sourcing this chemical calls for finding credible lab supply companies or chemical wholesalers with strict protocols. Well-known names like Sigma-Aldrich (now part of MilliporeSigma), Alfa Aesar, TCI America, and Oakwood Products often list it. Their websites put a heavy emphasis on technical data sheets and safety information. I have found that large distributors—ones that deal regularly with research labs, universities, and industry folks—expect their buyers to provide documentation showing legitimate use. This means a company name, research purpose, or reference from a licensed university. Some even ask for end-user declarations. They’re not simply tossing reagents into a shopping cart and calling it a day.
No reputable supplier will hand over controlled or hazardous chemicals without vetting the buyer. Due diligence takes center stage, not just to keep everyone safe, but to stay on the right side of regulatory agencies. Major players follow rules from agencies like the Drug Enforcement Administration (DEA) in the U.S. or European Chemical Agency (ECHA) in the EU. Some countries limit who can buy specific lab chemicals because of their possible misuse. If the application ties to university research, the ordering department usually manages the paperwork. If you run a small firm, prepare supporting documents and purchase records.
Buying from established companies comes with another plus: purity and documentation. In my own research projects, purity levels and material consistency made all the difference. Certificates of Analysis (CoA) prove essential, backing up supplier claims about chemical composition. Quality-control failures slow progress in the lab and can spark safety issues in larger applications. Unregulated or gray-market sellers miss these controls, creating more risks than bargains.
Some online sales platforms might show listings for 3-Methyltetrahydrothiophene 1,1-dioxide, but I warn against chasing what looks cheap or easy. Unauthorized sellers, sketchy websites, and informal forums often skip compliance, documentation, or even real product delivery. Law enforcement agencies have cracked down on chemical sales linked to misuse, so one risky purchase could lead to massive headaches—financial and legal. Trust comes from experience, not price alone.
The search for a specialized chemical like this boils down to navigating safety, legality, and scientific quality. Reputable chemical suppliers and distributors carry the right stock, ask the right questions, and deliver consistent batches. Solutions live with companies and organizations that have a real-world footprint and a reputation built over years, not faceless storefronts. Experience keeps buyers safe and projects on track. That’s what I’ve learned from the lab bench and beyond.
| Names | |
| Preferred IUPAC name | 3-Methyl-1λ⁶-thiolane-1,1-dione |
| Other names |
3-Methylsulfolane 3-Methylsulpholane 3-Methyl-1,1-dioxotetrahydrothiophene |
| Pronunciation | /ˌθriːˌmɛθ.əlˌtɛt.rəˌhaɪ.drəˈθaɪ.oʊˌθaɪ.iːnˈwʌnˌwʌnˈdaɪˌɒk.saɪd/ |
| Identifiers | |
| CAS Number | 878-35-5 |
| 3D model (JSmol) | `CC1CCSC1(=O)=O` |
| Beilstein Reference | 1109286 |
| ChEBI | CHEBI:132959 |
| ChEMBL | CHEMBL135106 |
| ChemSpider | 20836 |
| DrugBank | DB08303 |
| ECHA InfoCard | 03cd3af6-58f1-4753-8a54-576410c13ec1 |
| EC Number | 212-196-7 |
| Gmelin Reference | 104505 |
| KEGG | C01862 |
| MeSH | D008717 |
| PubChem CID | 83157 |
| RTECS number | KN3675000 |
| UNII | 691M64T65C |
| UN number | UN3335 |
| CompTox Dashboard (EPA) | DTXSID1050865 |
| Properties | |
| Chemical formula | C5H10O2S |
| Molar mass | 150.23 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | sweet |
| Density | 1.275 g/cm³ |
| Solubility in water | slightly soluble |
| log P | -0.28 |
| Vapor pressure | 0.45 mmHg (25°C) |
| Acidity (pKa) | 13.9 |
| Basicity (pKb) | 7.90 |
| Magnetic susceptibility (χ) | -73.63×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.480 |
| Viscosity | 13 cP (20°C) |
| Dipole moment | 3.96 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 234.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –396.9 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -3591 kJ·mol⁻¹ |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS05 |
| Signal word | Warning |
| Hazard statements | H302 + H315 + H319 + H335 |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 77°C |
| Autoignition temperature | 230°C |
| Explosive limits | 5.5–8.6% (in air) |
| Lethal dose or concentration | LD50 oral rat 2630 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 1790 mg/kg |
| NIOSH | SN8795000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 3-Methyltetrahydrothiophene 1,1-Dioxide: Not established |
| REL (Recommended) | 10 mg/m³ |
| IDLH (Immediate danger) | IDLH: 50 ppm |
| Related compounds | |
| Related compounds |
Tetrahydrothiophene Tetrahydrothiophene 1,1-dioxide Sulfolane 3-Methylsulfolane |