Chemists started paying serious attention to piperidine back in the late 1800s, drawn by its presence in black pepper and its basic nitrogen atom sitting inside that familiar six-membered ring. It didn’t take long before researchers began tweaking the ring, sliding alkyl groups onto carbons here and there. The methyl group at position three changed how the molecule behaves, shifting its reactivity and boiling point, and people realized it worked as a special building block. Lab notes from the 1930s and ‘40s show researchers experimenting with substitution to find easier synthesis. Old patents from Europe and America reveal push for efficiency: fewer steps, higher yields, less fuss separating the final product. Over decades, sharper crystallographers and spectroscopists built up a precise understanding of its structure, clearing a path for industrial-scale production and deeper research into what makes 3-Methylpiperidine stand out from its siblings.
3-Methylpiperidine comes off the line as a clear, colorless to slightly yellow liquid. It packs the sharp, fishy odor typical of small amines, which more than one student has cursed after opening a bottle in a crowded lab. You’ll find it in multiple grades, from high-purity material for pharmaceuticals and specialty chemical synthesis, all the way down to bulk mixes intended for agricultural intermediates. Commercial suppliers often package it in steel drums, amber glass, or polyethylene, always aiming for a tight seal since it evaporates quickly and picks up water from air without complaint.
This molecule boils at about 137°C and melts near -52°C. That means most ambient conditions leave it as a liquid but a quick rise in temperature sends vapors up. Water dissolves it at moderate rates, though the amine group prefers organic solvents. Its density falls near 0.85 g/cm³, making it lighter than water, so spills float. The basicity comes from the nitrogen lone pair, which also explains its affinity for acids, eager to form thick, oily salts. Methyl substitution steers both chemical behavior and physical feel: compared to plain piperidine, volatility changes, and the electron distribution over the ring makes certain reactions more or less favorable.
Manufacturers usually guarantee a minimum purity, quoted as 98% or higher, confirmed by gas chromatography or NMR. Labels highlight the CAS number (626-56-2), alongside storage information: avoid heat, sunlight, and open flames. Safety Data Sheets warn of corrosivity and vapor hazards, plus a reminder about proper ventilation. Barrels or bottles carry hazardous material symbols: flammable liquid, toxic if inhaled, hazardous to aquatic life. Buyers keep an eye on these specifications, because pharmaceutical or electronic applications require nearly absolute purity with trace metals and other amine impurities tightly restricted.
The most common approach to making 3-Methylpiperidine starts with a cyclization reaction. Chemists use 1,5-diaminopentane, teasing the molecule into a ring with catalysts like Raney nickel under hydrogen pressure. Another route tackles the job by alkylating piperidine with a methyl halide, but that path brings headaches with isomer separation and side reactions. The first method, though, pops out a product where the methyl sits exactly where you want it, pushing the yield higher and slashing the work of cleaning up later. Large plants rely on continuous-flow reactors, keeping conditions just so — enough pressure, the right temperature, steady supply of reactants — which makes large batch runs possible without snags.
Different chemists see different opportunities in 3-Methylpiperidine’s structure. The amine group at the heart of its chemistry snags alkyl groups in acylation reactions, reacts with acids to form salts, or acts as a nucleophile in substitutions. Electrochemical and catalytic hydrogenations reshape its ring, sometimes chopping off the methyl group or adding other side chains. Oxidation can target the ring or the methyl, steering products toward N-oxides or open-chain fragments. Medicinal chemistry teams often add protecting groups or swap out other hydrogens, using the compound as a scaffold for larger, bioactive molecules. Every reaction faces the question of selectivity – controlling which hydrogen or carbon reacts, since small changes can send chemists down the wrong synthetic road.
Chemists might call this compound 3-Methyl-hexahydropyridine, or just N-Methylpiperidine, but that latter name sometimes confuses it with N-methyl substituted analogs, so it’s best to confirm structures if there’s doubt. In some catalogs, you might see 3-Methylpiperidin or simply “3-MP.” Every label leads back to the CAS number, the universal fingerprint, even if regional suppliers coin their own trade names. International standards encourage unambiguous identification, especially for substances with multiple industrial applications spanning pharma, agriculture, and polymers.
3-Methylpiperidine does not play nicely with mucous membranes or skin, burning and causing redness or swelling on contact, while the vapors bring headaches and nausea. Proper handling needs gloves resistant to amines — nitrile or neoprene preferred — plus goggles and a fume hood. Storage goes in a cool, well-ventilated room, away from acids and oxidizers. Fire risks pop up thanks to low flash point (26°C), so even a warm draft might release enough vapor to ignite near a spark. Most labs keep spill kits with absorbents rated for organic amines, plus emergency showers for direct exposure. Operators must train on emergency protocols, and in industrial settings, safety automation—gas detection, fire suppression, vapor containment—backs up every process.
You’ll find 3-Methylpiperidine threaded through the pharmaceutical industry, especially in the construction of antihistamines, analgesics, and cognitive enhancers. Its ring suits drug candidates where nitrogen boosts activity or shifts distribution in the body. Outside pharma, agrochemical developers use the amine as a building block for plant-protection compounds — herbicides, insecticides, and growth regulators. Some pigment and polymer specialists use it to create specialty resins and coatings, taking advantage of its basicity and ready chemical reactivity. Even in analytical chemistry, people use it to derivatize certain analytes, improving detection or separation in chromatography.
Current research branches along a few main lines. Synthetic chemists work on greener, more selective routes for ring closure or methyl placement since cleaner reactions mean fewer downstream headaches. Academics also look at alternative feedstocks to cut reliance on petroleum-based precursors, exploring bio-based diaminopentanes and renewable hydrogen sources. In drug discovery labs, researchers tag derivatives with fluorescent or radioactive labels, probing metabolic pathways and receptor binding. Teams at national labs investigate its fundamental physical properties, refining computational models that predict how structures like 3-Methylpiperidine interact with enzymes, membranes, or catalysts. The hope: new medicines, faster production, and lower costs with less waste.
Animal testing over the decades shows that 3-Methylpiperidine carries moderate to high acute toxicity. Rats and mice exposed to its vapors display respiratory distress, and oral intake drives rapid systemic effects—tremors, ataxia, sometimes fatal at higher doses. Chronic exposure studies find liver and kidney effects, though less pronounced than with some other cyclic amines. Environmental studies show the compound breaks down somewhat in soil and water, but concerns about aquatic toxicity keep regulators cautious. Regulatory limits follow these findings, setting low occupational exposure ceilings and requiring personal protection in plants and research settings. New studies search for long-term impacts on DNA or cell metabolism, relevant for any future medical applications or occupational risks.
Looking forward, demand for 3-Methylpiperidine seems poised to grow as custom drug synthesis ramps up and farmers push for next-generation agrochemicals. Automation and process intensification promise safer, more efficient production, possibly allowing for on-demand synthesis closer to the point of use. Meanwhile, the search for new bioactive molecules almost guarantees more creative chemistry around the piperidine ring, with computer-aided design and high-throughput screening accelerating the hunt for valuable analogs. Green chemistry advances may shrink its environmental footprint over time, thanks to catalyst recycling and renewable raw materials. Regulatory frameworks will likely tighten, especially as new toxicity and exposure data appear, underscoring the need for ongoing vigilance and better protective measures in every facility working with this versatile amine.
I’ve always found it curious how certain chemicals hardly ever make headlines, yet their impact quietly shapes entire industries. 3-Methylpiperidine comes from that quiet, behind-the-scenes club. You won’t see it on billboards or hear it tossed around in cocktail party chat, yet for anyone who’s worked near a chemical plant, pharmaceutical lab, or pesticide facility, its name sparks clear associations. Most folks have never heard of it unless they’ve juggled reagents while troubleshooting factory batch issues, but its role runs deeper than you’d guess from a textbook summary.
Years back, I spent time shadowing a team developing crop protection agents. Their projects involved long hours sorting through compounds to boost resistance or manage pests. Here, 3-Methylpiperidine showed up as a building block in synthesis. It’s an organic molecule, shaped by its ring structure and attached methyl group, which makes it useful for piecing together more complex products. The agricultural world leans heavily on these basic chemicals. Pesticides, herbicides, fungicides — not a season goes by without the need for new options. This compound fits into that picture as a key intermediate. Without it, that chain snaps, and suddenly projects stall and costs spike.
Pharmaceutical research also draws on it. Next time you unwrap a prescription for antihistamines or drugs that knock out cold symptoms, trace the history back to the research bench. Chemists often use 3-Methylpiperidine while designing molecules that fit receptor targets in human bodies. The goal is to tweak a base chemical so it blocks a reaction or slows down a process causing illness — sometimes that tweak starts with a humble ring building block like this one.
My personal run-ins with 3-Methylpiperidine weren’t always pleasant. The compound’s notorious for its strong, ammonia-like odor. Even with years mixing batches, I’d catch a whiff and instantly want to air out the space. It’s flammable and can cause chemical burns if spilled. This smells like a minor issue, but safety officers will say otherwise if storage and waste handling get sloppy. Learning that lesson the hard way — after an accidental spill — sticks with you much longer than any item from a safety checklist. The warning labels on its containers exist for a reason.
Waste management poses another worry. Runoff from labs or factories shouldn’t just head straight into the local wastewater. Local regulators keep a close watch on disposal practices with compounds like this to protect waterways and public health. I’ve seen research teams take pride in developing greener byproducts and alternate routes for producing essential chemicals, cutting back hazardous waste at the source. Those efforts rarely grab headlines, but they chip away at long-term risks.
3-Methylpiperidine’s usefulness brings up an old dilemma: society relies on specialized chemicals, but how do you keep use both safe and sustainable? Sharper controls over storage and waste make sense, but improvements also come from shifting toward greener chemistry. Some scientists work on making intermediates like this from renewable feedstocks, not just oil-based routes. It’s not always the cheapest path, but reducing hazards matters. Chemical industries, from pharmaceuticals to farming, have plenty of incentives to get creative and trim down their reliance on harsh synthesis methods whenever possible. Employees win, so do downstream neighbors, and so does anyone who values long-term clean environments.
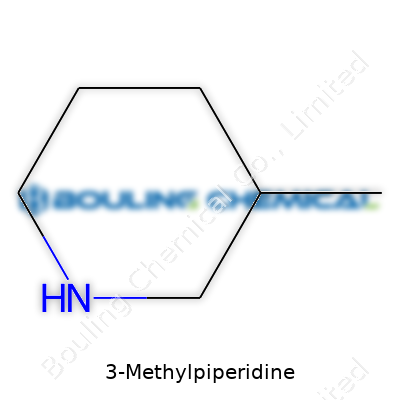
Take a walk through an organic chemistry lab, and you’ll probably smell something sharp and fishy—that could be a piperidine derivative, maybe even 3-Methylpiperidine. Chemists give things plain names, but 3-Methylpiperidine tells you a lot already: a piperidine ring has six atoms, mostly carbon, but with a nitrogen. The “3-methyl” just means a single carbon side chain sticks off the third spot. The chemical formula, C6H13N, captures that perfectly—six carbons, thirteen hydrogens, and a nitrogen atom.
Draw out its structure, and the shape reveals something that looks like a hexagon missing a side. Picture a lazy chair—five carbons, then a nitrogen, lined up to form a ring. Stick an extra carbon (the methyl group) onto the third carbon away from the nitrogen, counting in a circle. That bump changes everything, from how the molecule reacts to how it smells and feels. Small changes in these ringed compounds often tweak the chemical’s properties far more than you’d guess.
From my own time around organic syntheses, I’ve seen how molecules like 3-Methylpiperidine show up in more places than people expect. Because that extra bump on the ring bends the molecule slightly, it interacts differently with other chemicals than plain piperidine does. This property impacts everything from pharmaceuticals to the stuff used in rubber manufacturing or polymer additives.
Medicinal chemists spend a lot of effort swapping out little side groups on rings like piperidine just to see if drugs will behave better—being slightly less toxic, working a little longer, or finding the right mix of potency and safety. That methyl on the third carbon doesn’t sound like much, but it sets up a pattern that could open pathways for new compounds. In the world of drug discovery, small changes mean the world.
Working with piperidines—the plain kind or the methylated versions—means dealing with strong odors and, often, some mild toxicity. I’ve seen folks in the lab double glove and crack open extra windows just to keep the room breathable. 3-Methylpiperidine follows that same pattern: a jab to the nose, skin and eye irritation, and the need to keep it off your hands. Its strong odors sometimes stick in ventilation systems for days. Places that deal with these chemicals regularly invest in good fume hoods and plenty of training.
Issues pop up around safety and environmental handling more than anything else. Most regulations ask for careful storage in tightly sealed bottles, away from anything acidic or oxidizing. Spills might seem minor, but a little goes a long way to make a room unpleasant fast. Proper disposal and quick cleanup routines help keep messy accidents to a minimum.
Safer ways to use and dispose of amine derivatives like 3-Methylpiperidine deserve more attention. Labs and factories already switch to less toxic materials where possible, and new methods pop up regularly. Adopting greener syntheses—catalysts that use less hazardous waste or solvents—can keep people safer and help the environment.
As research pushes forward, these chemical tweaks teach us not just about one molecule but about a whole family of compounds. 3-Methylpiperidine may not sound glamorous, but it stands at the crossroads where small changes matter—a living example of chemistry’s power to shape what we make and how we handle it.
3-Methylpiperidine belongs to that group of chemicals that don’t make headlines until something goes wrong. Most folks haven’t heard of it, but those who work with it know: this stuff means business if ignored. Holding a sharp smell, catching fire easily, and irritating skin or eyes, it demands real respect in any work environment.
I once watched a seasoned lab tech get complacent with a similar chemical. A quick spill, a distracted moment, and sure enough—his skin snapped red and raw. Ammonia-like fumes filled the air, burning the nose even from a distance. That day stuck with me. Gloves, goggles, and lab coats aren’t optional extras; they make the difference between going home safe or heading to urgent care. With 3-Methylpiperidine, the risks run from nasty eye burns to potentially longer-term lung trouble.
Flash points matter. 3-Methylpiperidine catches fire at around 6°C—way below room temperature in many places. Picture summertime with no AC, electrical sparks from old wiring, or just a lazy afternoon with somebody lighting a cigarette. It’s tinder, plain and simple. Storing it in a flammable-proof cabinet, keeping it far from heat or open flames, and following those “no smoking” signs become non-negotiable rules. One small lapse changes the story from routine workday to fire brigade scramble.
Putting this chemical in a cool, well-ventilated spot sounds obvious, until coworkers cough in stuffy storerooms without fans or windows. I’ve seen containers heat up on a sunlit shelf, and fumes gather where nobody thought about airflow. Sadly, those “just for a while” storage choices turn minor leaks into major headaches. A tight lid, incompatible chemicals kept apart (no acids nearby, thank you), and good labeling can stop expensive mistakes before they start.
No one likes cleaning up after a spill, but a little planning saves chaos down the line. Absorbent materials, ready on the shelf. Spill kits in reach, not buried in a locked office. Clear instructions posted, so nobody fumbles. Over time, routines like these build muscle memory—and turn panicked confusion into quick, effective action. I’ve seen crews that prepped well handle small spills in minutes, protecting everyone in the area.
Many workplaces hand over bottles and hope for the best, but training changes the outcome. Clear explanations, real-life examples, and regular drills put everyone on the same page. Questions about “what if?” don’t sound paranoid anymore; they reveal holes in the system that actually get fixed. It shouldn’t take an accident for bosses to listen or invest in better gear. The cost of preparedness never outweighs the human toll of shortcuts.
Nobody wants to be the cautionary tale in a training slideshow. Good habits and real attention keep people safe, not just the rulebook. With 3-Methylpiperidine, the basics matter—and learning from other people’s experience is much easier than recovering from a bad day.
3-Methylpiperidine doesn’t come up in everyday conversation, but it quietly plays a role in chemical labs and certain manufacturing circles. This compound lands in the “amines” family, which puts it among chemicals that can carry a strong smell and sometimes a punch in terms of hazards. Its uses tie deeply into making pharmaceuticals, crop protection products, and specialty chemicals. For those outside of chemistry, bottles of this stuff could seem fairly nondescript, but its risk demands a closer look.
A whiff of 3-Methylpiperidine tells its own story: sharp, ammonia-like, almost enough to knock you back. That’s not just a warning to your nose. Even exposure at low levels can irritate eyes, skin, and lungs. Once this compound gets onto skin or into the air, effects often follow fast—redness, burning, coughing, even headache. Handling larger amounts raises the risk quickly. If swallowed or splashed directly into the eyes, 3-Methylpiperidine brings far worse consequences: serious chemical burns and possible damage to tissues that won’t bounce back in a hurry.
Numbers cut through the confusion. Lab tests have pinned the acute oral toxicity (LD50 in rats) at about 266 mg/kg. That lands it in the “toxic” range according to globally recognized safety charts. Breathing in concentrated vapors or mist can escalate from simple discomfort to respiratory distress. Extended exposure, even at low levels, can slide into chronic problems: repeated skin contact may cause dermatitis, and there’s solid evidence for liver and kidney strain in animal studies. This isn’t just theory—the rules around 3-Methylpiperidine require specialized safety gear, proper ventilation, and strict controls in every commercial lab or plant.
It’s easy to forget just how dangerous some chemicals can be when bottles are lined up on a shelf, but a single spill or careless transfer with 3-Methylpiperidine can trigger an emergency. Most people handling it haven’t chosen to take that risk at home; this isn’t the kind of substance you’ll find in cleaning products or over-the-counter goods. The risks come into play at points in the supply chain where safety culture can mean the difference between uneventful work and a trip to the hospital.
Problems only multiply if organizations don’t take safety seriously. This is where on-the-ground experience changes the story. Proper fume hoods and closed transfer systems go from “nice to haves” to essentials. Gloves, goggles, and lab coats give a first line of protection, and that’s just the start. Regular safety drills, up-to-date documentation, and clear emergency procedures bring peace of mind for workers. It’s not enough to know a hazard exists; acting on that information every day actually makes a difference.
Nobody expects everyone to understand every risk from memory. Safety Data Sheets exist for a reason, and staying current with regulations helps catch new research or risk factors that might show up. In some cases, switching away from 3-Methylpiperidine, when a safer alternative works just as well, shows respect for people on the front lines. Investments in safer processes and updated training often pay off many times over, not just in regulatory compliance but in real-world health.
The case with 3-Methylpiperidine draws a clear line: knowledge and preparation matter more than almost anything else. Real safety doesn’t live in paperwork. It takes root in the big and small actions people take every shift—treating every step with care, keeping an eye on those around them, and refusing to cut corners. For those who spend time near this compound, staying healthy depends not just on what they know, but what they’re willing to do every day.
3-Methylpiperidine isn’t a chemical you meet walking down the street, but in a lab, you can’t really avoid its bite. On the market, the most common purity on offer lands at about 98%. For scientists worried about byproducts in their reactions or folks working in pharmaceutical synthesis, the jump to 99% brings more peace of mind at a price. Every percent counts, especially in work where a rogue impurity can kill a whole project or force hours of troubleshooting.
You usually see these percentages on certificates of analysis that come with each batch. In my own work, reading that little slip of paper feels like rolling the dice – but most reputable chemical suppliers back up their numbers with gas chromatography or NMR results, double-checking what’s really in the drum or bottle. Anything below 98% feels like asking for trouble, unless you’re just using it for a teaching demo and not some sensitive step in making a new molecule.
Let’s talk packaging. 3-Methylpiperidine stinks, literally; it’s sharp and fishy, and the odor gets everywhere if you’re not careful. The packaging options usually include amber glass bottles with tight plastic seals for lab-scale needs, typically in weights like 100 grams, 250 grams, or half-kilogram packs. I’ve run into plenty of headaches with plastic bottles, and leaks are a real risk. Glass saves benches and noses from disaster.
With quantities larger than a kilo, suppliers move up to metal drums or HDPE jerrycans. These come with vented caps and lining to avoid chemical reactions with the container. Those vented caps make a difference if you’re dealing with something that can build up pressure, which 3-Methylpiperidine is prone to do as the temperature climbs.
Transporting larger amounts means living with annoying paperwork—hazard class for transport, written approval, material safety data on hand at all times. I’ve had shipments blocked or returned for missing a sticker or an outdated hazard symbol, and that delays research, eats funding, and sours collaborations all at once.
Once the bottle or drum lands in your hands, the purity and packaging stop being background details. Poor packaging leads to evaporation, leaks, and exposure – all disastrous, since this chemical can cause skin irritation and has a nasty odor threshold. In one hellish incident, I opened a poorly sealed drum and found half the lab complaining about the smell. Next time, I asked suppliers for upgraded seals, triple packaging, and vapor barriers.
Choosing the right size matters too. In a big pharma setting, you might run through a 25-liter drum in a week; in a smaller campus lab, we nurse the same 100-gram glass bottle for a year. Buying bigger than you need just invites degradation or regulatory nightmares.
Behind every purchase of 3-Methylpiperidine, someone is betting on reliability and clear labeling. I always prefer suppliers that don’t skimp on documentation: batch analysis, packing date, full breakdown of all detected impurities, and hazard details clear as day. Inconsistencies between labels and supplied paperwork make my job a guessing game – and almost always force me to conduct more purity testing than I wanted to in the first place.
For researchers tired of running into issues, two fixes stand out: buy only the amount needed for active projects (not future guessing) and demand full transparency on purity and stability. It saves time, budget, and reduces headaches down the line. Packaging, done right, saves relationships between people and labs. That’s a small detail that matters much more than most people think.
| Names | |
| Preferred IUPAC name | 3-Methylpiperidine |
| Other names |
3-Methylpiperidine Piperyl methyl 3-Methylhexahydropyridine |
| Pronunciation | /ˌθriːˈmɛθɪlpaɪˈpɛrɪdiːn/ |
| Identifiers | |
| CAS Number | 626-56-2 |
| 3D model (JSmol) | `3d7e322210937233b1e002c781432a15` |
| Beilstein Reference | 1209239 |
| ChEBI | CHEBI:51169 |
| ChEMBL | CHEMBL15362 |
| ChemSpider | 15365 |
| DrugBank | DB04211 |
| ECHA InfoCard | ECHA InfoCard: 100.007.975 |
| EC Number | 203-648-2 |
| Gmelin Reference | **7939** |
| KEGG | C06359 |
| MeSH | D010006 |
| PubChem CID | 7906 |
| RTECS number | EK3675000 |
| UNII | 2U83BQN57T |
| UN number | UN2386 |
| Properties | |
| Chemical formula | C6H15N |
| Molar mass | 101.19 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | fishy |
| Density | 0.818 g/mL |
| Solubility in water | miscible |
| log P | 0.94 |
| Vapor pressure | 3.4 kPa (at 20 °C) |
| Acidity (pKa) | 11.2 |
| Basicity (pKb) | 2.84 |
| Magnetic susceptibility (χ) | -7.73 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.432 |
| Viscosity | 0.778 cP (20°C) |
| Dipole moment | 2.25 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 203.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -44.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4246.6 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS05, GHS06 |
| Pictograms | GHS02,GHS06,GHS05 |
| Signal word | Danger |
| Hazard statements | H225, H302, H314, H411 |
| Precautionary statements | P280, P261, P271, P304+P340, P312, P305+P351+P338, P301+P330+P331, P405, P501 |
| NFPA 704 (fire diamond) | 3-3-0 |
| Flash point | 32 °C (90 °F) |
| Autoignition temperature | 315 °C |
| Explosive limits | 1.1% - 7% |
| Lethal dose or concentration | LD50 oral rat 266 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat 266 mg/kg |
| NIOSH | STY70150 |
| PEL (Permissible) | PEL: 100 ppm |
| REL (Recommended) | 0.2 ppm |
| IDLH (Immediate danger) | 400 ppm |
| Related compounds | |
| Related compounds |
2-Methylpiperidine 4-Methylpiperidine Piperidine 3,5-Dimethylpiperidine 2,6-Dimethylpiperidine |