3-Methyl thiophene-2-carboxylic acid first turned up in research circles in the early decades of sulfur-containing heterocyclic chemistry. Back in the post-war years, chemists explored thiophene rings as building blocks for dyes, drugs, and agricultural chemicals. Growing interest in thiophene derivatives took off during the 1960s, thanks to new synthetic routes and the promise of diverse biological activity. Scientists recognized that modifying the thiophene scaffold—specifically, introducing a methyl and a carboxy group—could open doors to novel pharmaceuticals and crop protection agents. It often appeared in patent filings through the 1980s and beyond, as every tweak in structure meant the difference between an inactive compound and a blockbuster candidate. The story of this molecule sits within a wider tale: chemists always chasing that balance of function, feasibility, and fresh chemistry.
3-Methyl thiophene-2-carboxylic acid fits into an expanding library of heterocyclic acids used for downstream synthesis. With a methyl group attached to the third carbon of the thiophene ring and a carboxylic acid at the second, it carries unique reactivity thanks to the interplay between those positions. This arrangement lets it serve as a versatile intermediate—often used in constructing active pharmaceutical ingredients, agrochemicals, and organic electronic materials. It’s not the flashiest compound on a bench, but its structure opens up a toolbox of transformations. Research labs and industrial platforms alike often keep this carboxylic acid in stock for building complex molecules, exploiting its handle for both further derivatization and conjugation.
At room temperature, 3-methyl thiophene-2-carboxylic acid tends to appear as a white or off-white crystalline powder, although slight yellowing isn't rare due to minor oxidation. It doesn't dissolve well in water but dissolves readily in many polar organic solvents, including ethanol, acetone, and dimethyl sulfoxide. Its melting point typically falls between 90°C and 120°C, reflecting strong crystal lattice forces typical for carboxylic acids. The molecule weighs in at about 142 g/mol, with a noticeable sulfur odor—a hallmark of thiophene compounds that still catches people off guard in the lab. The aromatic thiophene ring shows significant stability, fairly resistant to reduction but eager for a range of substitution reactions. Acid dissociation constants (pKa) match what you’d expect from a benzoic acid derivative, making it suitable for use in pH-sensitive syntheses.
Specifications for 3-methyl thiophene-2-carboxylic acid target purity above 98%, often confirmed by high-performance liquid chromatography and NMR analysis. Small traces of related thiophene derivatives or minor organic acids appear as impurities in off-spec lots. Manufacturers label containers with standard chemical names, hazard pictograms, and QR codes to track batch history. Labels include UN numbers and recommendations for storage—typically sealed in dry, cool conditions out of sunlight. Experienced chemists and handlers appreciate clear labeling, since the smell alone doesn’t always reveal what’s inside a flask. Knowing the grade—analytical, technical, or high-purity—matters a lot depending on downstream use, especially when the next steps move toward regulated applications like pharmaceuticals.
Several routes lead to 3-methyl thiophene-2-carboxylic acid, but the most common starts with the thiophene ring itself. Chemists often employ Friedel–Crafts acylation, introducing a methyl group at the 3-position with methyl halides and Lewis acid catalysts. Carboxylation typically follows, sometimes utilizing metal-catalyzed carbonylation or transition metal-promoted direct functionalization. Another reliable path runs through Vilsmeier–Haack formylation, introducing a formyl group, followed by methylation and subsequent oxidation to the carboxylic acid. Each step requires strict control of temperature, solvents, and reaction times, as over-alkylation or ring degradation can lure the impatient. Scale-up in industry brings its own tweaks, such as flow chemistry adaptations and greener solvents, but the core logic remains: control the position, control the product.
The molecule’s carboxylic acid group offers easy access to esters, amides, and acid chlorides, making it a favorite for coupling reactions. Standard peptide synthesis methods attach this acid to amines or alcohols with carbodiimide or coupling agents. The methyl group, set at the 3-position, steers electrophilic aromatic substitution toward remaining open carbons, guiding functionalizations away from unwanted side products. Oxidation of the methyl can yield the corresponding aldehyde or alcohol, providing even more variety for organic synthesis. On the thiophene ring, halogenation, nitration, and sulfonation open the door to further utility in medicinal or polymer chemistry. These modification pathways supply both researchers and manufacturers with a range of derivatives, many of which display unique behavior in the body, in plants, or in electronic materials.
Catalogs and journals call 3-methyl thiophene-2-carboxylic acid by several names, with “2-carboxy-3-methylthiophene” often top of the list. Older literature sometimes refers to it as “β-carboxy-3-methylthiophene,” reflecting historical nomenclature conventions. Product listings show it under “3-Methyl-2-thiophenecarboxylic acid” or, more rarely, “3-MT-2-CA.” These variations stem from the standardization efforts of IUPAC through the 20th century. Recognizing synonyms prevents mix-ups when ordering or interpreting older research: more than one chemist has ordered the wrong acid because a vendor used local labeling standards. CAS numbers provide a consistent anchor—one code to rule out ambiguity in purchasing, inventory, or regulatory compliance.
Handling thiophene derivatives means you work with volatile organic compounds, some of which bring moderate inhalation risks and skin irritancy. 3-Methyl thiophene-2-carboxylic acid falls into the “caution, but not high alert” category. Users wear gloves, eye protection, and work in well-ventilated spaces or fume hoods. Safety data sheets stress the importance of avoiding prolonged skin contact or accidental ingestion, as metabolic pathways for thiophene compounds sometimes yield reactive metabolites. Storage in airtight containers slows degradation and reduces the chance for moisture-induced hydrolysis or mold growth. Disposal methods stick to licensed solvent waste handling, with local permits and documentation needed for large-scale streams. Major chemical suppliers conduct extensive hazard assessments, and many university labs adopt institutional protocols above the regulatory minimum.
This compound shows up most in organic synthesis and pharmaceutical R&D, acting as both a building block and a test substrate. Its tailored reactivity and spatial orientation on the thiophene ring help medicinal chemists fine-tune lead compounds for drug discovery. Agrochemical firms use it in screening programs, looking for new herbicides and fungicides. Materials scientists investigate its role as a monomer or additive for organic conductors, OLEDs, and sensory polymers, seeking high-performance properties for electronics. While it doesn’t headline many commercial products directly, it underpins research pipelines — giving both industry and academia the tools to go after tougher scientific and practical problems.
University and industry labs continue to draw on 3-methyl thiophene-2-carboxylic acid for exploratory research, linking it with new compounds that show promise as clinical candidates or advanced materials. Research articles track its reactivity across cross-coupling, late-stage functionalization, and bioisosteric replacement strategies. Funding often supports screening programs that convert this acid into larger libraries for biological assays. In the last decade, green chemistry innovations have shifted attention to cleaner synthesis methods—minimizing hazardous reagents, reducing energy use, and recycling solvents. Collaborative projects between chemical suppliers and research teams work toward more sustainable supply chains and broader regulatory approval, clearing roadblocks on the path from bench to large-scale application.
Animal and cell studies of thiophene and its derivatives make up much of the toxicity literature. 3-Methyl thiophene-2-carboxylic acid itself hasn’t shown the acute toxicity common to more reactive sulfur-containing aromatics; it generally features low to moderate oral and dermal toxicity in lab animals. Chronic exposure data remain limited. Some studies indicate mild mutagenicity at high concentrations, prompting an emphasis on PPE and exposure controls. Researchers continue to investigate its metabolic fate—especially in mammals—since oxidation may produce electrophilic intermediates that could stress liver enzymes and induce oxidative damage. Experience in the research lab has shown that, treated with respect and correct safety practices, this molecule presents manageable risks compared to many organic synthons with greater volatility or bioactivity.
As organic synthesis moves into the era of precise molecular construction, 3-methyl thiophene-2-carboxylic acid will remain a go-to starting point for new pharmaceuticals and specialty polymers. With the demand for sustainable and efficient synthetic methods rising, interest in streamlining its production grows. Researchers focus on catalysis, flow chemistry, and bio-based starting materials to reduce environmental impact. As medicinal chemistry projects demand new scaffolds for treating diseases that resist existing drugs, the unique combination of thiophene and carboxylic functionality allows rapid expansion of compound libraries. Advances in computational chemistry enable tight prediction of its reactivity, accelerating the design of new derivatives. In the landscape of future chemical manufacturing, this compound stands to play an outsized role, supporting a shift to molecules that hit precise therapeutic, agronomic, and materials targets—and shaping the next chapters of chemical innovation.
Think about 3-Methyl Thiophene-2-Carboxylic Acid and you’ll see a ring-shaped molecule, combining both complexity and accessibility. It’s built on a thiophene core. Thiophene itself forms a five-membered aromatic ring—a sort of cousin to benzene—except a sulfur atom takes the place of one of the carbons. A methyl group comes off the third carbon in this ring, and a carboxylic acid group attaches at the second. Chemists would write its structure as a five-pointed ring, numbering the atoms starting from sulfur, in a clockwise fashion. The formula reads C6H6O2S when you tally up all the atoms.
Structure isn’t a dry detail. My personal experience working in a university lab taught me how even small changes, like shifting a group to a different carbon, can send a compound’s reactivity off in a new direction. That methyl group at position three brings a subtle balance—it adds electron density, slightly modifying how the molecule interacts with other chemicals or metal ions. The carboxylic acid group at position two makes it possible to link up with proteins, metals, or to serve as a starting point for further chemical transformations.
Industries designing dyes, pharmaceuticals, or materials use molecules like this for a reason. A carboxylic acid group acts like a handle, giving chemists flexibility for making esters, amides, or more exotic products. The thiophene ring itself opens doors: In electronics, sulfur in the ring helps deliver stability and conductivity. Altering side groups on these rings creates materials for use in organic semiconductors and sensors.
From a bioscience perspective, acids like this can serve as chemical building blocks to mimic natural products or interface with enzymes. Working with carboxylic acids meant easy conversions—reacting with alcohols for esters or with amines for making amides. In drug research, those connections can mean everything for activity or safety in the body. Small tweaks, whether a methyl group or a shift on the ring, play a role in absorption, metabolism, or even how a compound tastes.
The science behind this structure comes from years of synthetic work and crystallography. X-ray crystallography gives a clear window into the molecule: which atom sits where, how the bond angles differ slightly from textbook diagrams because of ring strain, and how neighboring atoms influence each other through resonance. I learned in my studies how results sometimes surprise: predicted reactivity based on drawing alone sometimes fails without seeing the real, three-dimensional shape. In those cases, hands-on lab experience pointed the way to real-world chemistry, not just equations.
The future doesn’t stop with one molecule. By understanding 3-Methyl Thiophene-2-Carboxylic Acid on an atomic level, young chemists, industries, and researchers can push solutions forward—safer drugs, better electronic materials, and even greener manufacturing. The lessons from this ring-and-tail structure anchor every project: think deeply about the connections, imagine each atom, and build from there.
3-Methyl Thiophene-2-Carboxylic Acid keeps showing up in labs searching for the next big medicinal breakthrough. Its unique thiophene core lets chemists build drugs that interact in precise ways with the human body. The pharmaceutical industry often chases small molecules like this because they can serve as critical links or “building blocks” in making more complex substances. People working on new antibiotics and anti-inflammatory agents rely on compounds with thiophene rings, drawn to their stability and ability to tweak how an active ingredient behaves in the body.
The process can take years, yet the right intermediate unlocks new classes of medicines. Having worked in a research team exploring drug analogs, I noticed how easily a small change in core structure, like that provided by 3-Methyl Thiophene-2-Carboxylic Acid, alters both safety and effectiveness. Safety and targeted action are the top priorities for regulators and drug developers today. As a result, substances with a proven track record and rich synthetic possibilities attract lasting attention.
Farm chemistry increasingly leans on heterocyclic compounds. Crops face threats from pests that adapt quickly, so scientists keep searching for new ways to break harm cycles. In this setting, 3-Methyl Thiophene-2-Carboxylic Acid plays a role as a “skeleton” for building pest control solutions. Most commonly, it enters the picture early in the molecule’s design, helping to anchor key features that knock out resistant insects or fungi. The thiophene structure catches the interest of chemists because it stands up well to weather and doesn’t break down before doing its job.
Regulators around the world ask for clear breakdowns of environmental impact. The structure of thiophene-based acids allows for testing variations in activity and breakdown speed, which is crucial when companies need options for different climates and farming methods.
There’s a growing idea that scent chemists borrow techniques from pharma and agro research. Some modern preparation of flavors and fragrances use small, sulfur-rich rings, like what’s in 3-Methyl Thiophene-2-Carboxylic Acid, to develop richer or more striking sensations. The chemical’s molecular makeup helps mimic earthy or roasted tones valuable in luxury perfumes.
Meanwhile, electronics makers look at thiophene molecules for how they transport electrons. Flexible displays, solar panels, and some sensors use these rings because they move charge well. I remember a project that tested modified thiophenes in a next-gen display—performance changes could be traced right down to single atoms swapped on the ring structure. That level of control opens up countless paths for energy solutions and smart device progress.
Across every use—drug research, crop treatments, electronics, or fragrances—traceability and purity take the spotlight. Labs demand certification proving the compound’s makeup. That means big investments in analytical testing and documentation. Safety remains central, both for researchers handling the substance and for anyone using end products, so supply chains now want full transparency.
Standards continue to get stricter. Companies develop systems to monitor and review each step, from raw material sourcing to shipment. In my experience, chemists choose suppliers with the best track record, even if it means paying a little more, just to avoid risks and delays. The synthetic versatility of 3-Methyl Thiophene-2-Carboxylic Acid promises more breakthroughs, but none of them matter if they don’t hit benchmarks for reliability.
People working in chemistry, pharmaceuticals, or advanced materials know that purity isn't a luxury. It makes the difference between a successful reaction and an expensive mess. I’ve seen research projects slow to a crawl because someone didn’t realize a 98% purity compound wasn’t enough. With 3-Methyl Thiophene-2-Carboxylic Acid, the difference between 97% and 99% purity can mean hours spent troubleshooting a reaction instead of moving onto data analysis.
Researchers pick their products based on intended use. Trace impurities—just one or two percent—can introduce conflicting signals in synthesis or analysis. A small impurity can throw off spectroscopy and chromatography readings, leading to false positives or missed products. Story after story floats around chemistry labs about someone discovering an unknown peak during NMR work, only to trace it back to an impure starting compound.
At scale, pharmaceutical and agrochemical companies demand defined quality specifications. Regulatory agencies like the FDA and EMA ask for data that leaves no room for ambiguity. They’ve flagged drugs due to impurity problems, sometimes with huge product recalls. Imagine investing months into a drug candidate, only to trace a problem back to contaminated 3-Methyl Thiophene-2-Carboxylic Acid.
Most suppliers sell this compound at a purity of 97% to 99%, checked by methods like HPLC, GC-MS, NMR, and elemental analysis. These numbers aren’t pulled out of thin air: strict lab protocols and reference standards make sure the stated value reflects reality. My own experience with supplier certificates tells me, always double-check the provided data using your own lab’s quality controls before trusting a label claim.
The difference between “97% technical grade” and “99% analytical grade” might seem minor until a sensitive step starts failing. Even 0.5% unknowns can catalyze side reactions, poison catalysts, or mess up yields. That’s the kind of headache nobody wants to explain to their PI or production manager.
People often skip over product specs in favor of price or delivery time. That shortcut usually catches up with them later. The few dollars saved on a lower grade batch could be wiped out by troubleshooting hours and scrapped materials. I learned early on that it’s smarter to raise budget questions before starting a project than justify an incomplete dataset later.
In my own work, running QC on every chemical first became a ritual. About one out of five times, suppliers’ purity claims didn’t quite match the numbers from our in-house tests. Sometimes the culprit was a little excess water or a bit of a manufacturing solvent left behind. Picking up on that early saves time, resources, and sometimes, safety incidents with reactive impurities.
To keep up with modern quality demands, labs and companies must invest in both reliable suppliers and regular in-house purity checks. Working with a trusted supplier, confirming each lot with HPLC or NMR, and documenting results creates a safety net. Metals, unknown byproducts, and residual solvents leave a fingerprint in these tests. Training new chemists to recognize the dangers of ignoring purity claims makes a long-term impact and protects reputations.
As the chemical industry moves forward, precise quality control on 3-Methyl Thiophene-2-Carboxylic Acid won’t just stay relevant—it will become more critical. Reproducible science and safe manufacturing hinge on trusting the quality of every starting material, every time.
Working with chemicals like 3-Methyl Thiophene-2-Carboxylic Acid means taking proper care to ensure safe storage. Experience teaches that small missteps in how chemicals are handled—especially with organic acids—can turn routine tasks into risky situations. This isn’t just about regulatory compliance; it’s about safeguarding employees, protecting property, and making sure every batch meets quality expectations.
Chemicals react to their environment, sometimes in ways that surprise even seasoned lab technicians. Storing 3-Methyl Thiophene-2-Carboxylic Acid in cool, dry conditions—preferably at room temperature or just a bit below—keeps it stable and reliable. Labs that keep the temperature around 20°C to 25°C find fewer issues with product degradation or unwanted reactions. High heat or freezing can both spoil its intended use. Even short exposure to elevated temperatures during summer or near sources like radiators can cause decomposition, throwing off experiments or hurting manufacturing yields.
Humidity plays a role too. If water vapor seeps into containers, it doesn’t just dilute the acid—it can help trigger side reactions or even spoil the container itself. That’s bad news for inventory, and reordering always disrupts schedules. Proper storage means using tight-sealing bottles made from compatible materials—ideally, amber glass—to block out both moisture and light.
People familiar with 3-Methyl Thiophene-2-Carboxylic Acid know that direct light—or even strong room lighting over many months—brings its own risks. Ultraviolet in sunlight especially can spark reactions in sensitive organic acids. Protecting the acid with amber glass, or by storing containers in opaque cabinets, limits these problems. Manufacturers recommend this for a reason: less degradation, more consistent results, greater safety.
Labels fade faster than you might think, especially in labs where solvent fumes or water splash around. Permanent, chemical-resistant markers do the job. Every bottle with 3-Methyl Thiophene-2-Carboxylic Acid needs clear, up-to-date labeling—name, date of receipt, and hazard symbols. It’s not just for convenience. Emergency responders depend on that information, and so do anyone working near the storage area.
I’ve seen labs where old stock sits hidden in dusty corners because nobody knows if it’s expired or still good. This invites expensive mistakes. A routine—monthly checks for outdated or damaged bottles—cuts down on accidents.
Some chemicals don’t play well together. Acids and strong bases, oxidizers, or reactive metals need their own spaces. Mixing storage invites fire or toxic gas leaks. Separate shelves or cabinets, each clearly marked, help avoid these risks.
Everyone who touches, transports, or stores 3-Methyl Thiophene-2-Carboxylic Acid needs real training, not just a memo. Walk-throughs on proper storage, spill handling, and emergency response make a measurable difference. It’s also important to review safety data sheets with every new shipment. Clear, accessible documentation builds a culture of safety that lasts longer than any inspection.
Keeping storage straightforward isn’t hard. Choose cool, dry, dark spaces; use tight containers and clear labels; avoid stacking acids with incompatibles. Regular checks and good training hold everything together. These habits build trust across teams and help every lab worker know they’re working in a place that values both safety and quality.
Sitting in front of a beaker or notebook, a chemist expects clear facts and straight-shooting instructions, especially when new chemicals roll in. 3-Methyl Thiophene-2-Carboxylic Acid may not stand out to those outside the lab, but anyone who handles organic sulfur compounds knows even simple molecules can bite if left unchecked. Some chemists stumble through vague material safety data sheets (MSDS), and that's where the problems start to stack up.
With 3-Methyl Thiophene-2-Carboxylic Acid, searching safety data can leave gaps. Routine chemical references won’t compete with thorough guides like Sigma-Aldrich’s or PubChem’s listings. Yet, even reputable sources sometimes produce blanket recommendations vaguely copied from similar organosulfur acids. The trouble comes from nuance—skin absorption risks, proper fume hood ventilation, and those chance instances when a minor splash becomes a week-long health problem.
Reading through a half-page of “avoid inhalation and contact” feels a lot like hearing a doctor say “eat better and exercise.” Handling sulfur-containing acids like this one, I’ve watched gloves degrade in days, and skin dry out from careless splashes. Yet, some safety sheets just slap on the general “irritant” label and call it a day.
Nothing replaces hands-on advice from someone who’s been splashed, dealt with nasty smells, or cleaned spills under tight deadlines. While most organic acids draw out the same “use PPE and fume hood” warnings, lab techs share more honest stories—the stray drop on a wrist, shortness of breath after a wide-mouth flask mishap, headaches from poorly ventilated benches. You can't ignore those tales if you want safe practice.
Safety advice lags behind reality, usually because labs use a chemical less than the big stuff—those with hundreds of gallons shipped per year. 3-Methyl Thiophene-2-Carboxylic Acid sits in that strange category, more likely for research than routine industrial use. This rarity means most MSDS and safety databases borrow lines from similar thiophene acids or whip up best guesses, sometimes missing key properties unique to this compound.
Reviews suggest it causes skin, eye, and respiratory irritation. Because it’s an organic acid, contact with bases or oxidizers could produce hazardous byproducts. Yet, there’s little reliable information on fire hazards, long-term health effects, or environmental impact, which leaves users in the lurch. If a spill occurs, guidelines say to just “collect and dispose” without spelling out what substances neutralize it best or how persistent its smell or residue proves in a typical workspace.
Relying on catch-all safety blurbs from chemical suppliers short-changes users. Researchers, chemical handlers, and safety officers all face higher risk without solid facts. Creating safety guides from scratch—including full PPE checks, spill scenario run-throughs, and practical control experiments in small batches—can fill blanks where standard MSDS leaves silence.
Academic institutions and companies should encourage chemists to document near-misses, minor exposures, and variations in glove and respirator effectiveness. Reporting these up the chain—then updating safety sheets—prevents other labs from learning lessons the hard way. The best protection doesn’t come from a database entry. It grows from a network that cares about keeping everyone healthy, grounded in science and real experience.
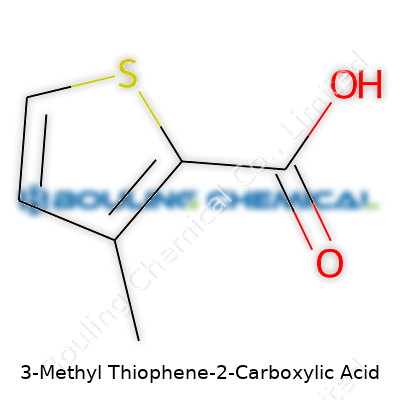
| Names | |
| Preferred IUPAC name | 3-methylthiophene-2-carboxylic acid |
| Other names |
3-Methyl-2-thiophenecarboxylic acid 3-Methyl-2-thiophene carboxylic acid 3-Methylthien-2-carboxylic acid 3-Methyl-2-thiophenecarboxylate acid 3-Methyl-2-thiophenecarboxylsäure |
| Pronunciation | /θriːˈmɛθ.ɪl θaɪ.oʊˈfiːn tuː kɑːrˈbɒk.sɪl.ɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | [16745-00-9] |
| 3D model (JSmol) | `3dmol__Mol__data_2D_mol__JSmol__MSD__PDB__Molfile__mol__C6H6O2S__JSMOL__Scc1ccc(C(=O)O)c1C` |
| Beilstein Reference | Beilstein Reference: 107230 |
| ChEBI | CHEBI:91227 |
| ChEMBL | CHEMBL16232 |
| ChemSpider | 178038 |
| DrugBank | DB04523 |
| ECHA InfoCard | 20-3-777393256 |
| EC Number | 87632-30-2 |
| Gmelin Reference | 55418 |
| KEGG | C18615 |
| MeSH | D04717 |
| PubChem CID | 115458 |
| RTECS number | GL9210000 |
| UNII | J9U219Y4X1 |
| UN number | UN3335 |
| CompTox Dashboard (EPA) | DJ6T0Q16V6 |
| Properties | |
| Chemical formula | C6H6O2S |
| Molar mass | 142.18 g/mol |
| Appearance | White to Off-White Solid |
| Odor | Strong odour |
| Density | 1.342 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | 1.7 |
| Vapor pressure | 0.0000727 mmHg at 25°C |
| Acidity (pKa) | 3.92 |
| Basicity (pKb) | pKb = 11.14 |
| Magnetic susceptibility (χ) | -24.1 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.565 |
| Dipole moment | 2.51 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 207.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -121.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -5562.6 kJ/mol |
| Pharmacology | |
| ATC code | Not assigned |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin irritation, causes serious eye irritation |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264; P270; P301+P312; P330; P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | > 120°C |
| NIOSH | NM9456000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 3-Methyl Thiophene-2-Carboxylic Acid is not specifically established by OSHA or other major regulatory agencies. |
| REL (Recommended) | 10 mg to 1 g |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Thiophene-2-carboxylic acid 3-Methylthiophene 3-Methylthiophene-2-carboxaldehyde 2-Bromothiophene-3-carboxylic acid 3-Ethylthiophene-2-carboxylic acid 3-Methylthiophene-2-sulfonic acid |