Chemists began working on the benzothiazole skeleton in the early twentieth century, searching for heterocyclic frameworks that could offer new reactivity and biological potential. Tinkering with methyl groups brought new avenues, and researchers quickly noticed that attaching a methyl to the third carbon influenced electronic effects and solubility. Laboratories across Europe and Asia contributed detailed notes on synthetic routes, basic reactivity, and structural verification. Over decades, refinements in the synthesis of 3-Methyl-1,2-Benzothiazole-1,1-Dione emerged, moving from mixed yields and crude isolation to more robust approaches using defined reagents and analytical confirmation with modern spectroscopy. Journals began highlighting derivatives as promising for dyes, pharmaceutical scaffolds, and as starting materials in both organic and medicinal chemistry.
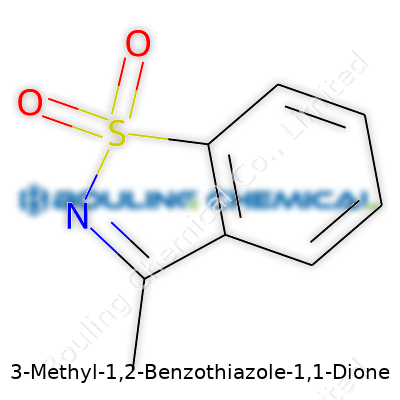
3-Methyl-1,2-Benzothiazole-1,1-Dione stands out as an aromatic sulfur-nitrogen heterocycle, where the dione moiety offers electrophilic character, and the methyl on carbon 3 subtly shifts both chemical behavior and physical properties. Chemists use this compound to access further substituted benzothiazoles and as an intermediate for dye compounds and biological evaluation. It’s gained attention for its reactivity in condensation and ring-closure reactions and as a platform from which to build libraries of new molecules. Many see it as a linchpin for synthetic advances in both academic and industrial settings.
This compound appears as a pale yellow to off-white crystalline powder. It dissolves modestly in polar organic solvents like acetone, DMSO, and DMF, while showing limited solubility in nonpolar media. At room temperature, it remains stable under dry conditions, though prolonged exposure to moisture or high temperatures can trigger hydrolysis or decomposition. The molecule’s structure grants it distinct melting behavior, typically melting in the 130-145°C range, with decomposition occurring shortly above this thermal window. Its UV-visible spectroscopic signature reflects the conjugated aromatic core, and IR spectra reveal sharp patterns for the sulfonyl, carbonyl, and ring vibrations. Chemical stability under neutral and mildly basic conditions supports its use as a reliable intermediate, yet strong acids or bases break it down or open the benzothiazole ring.
Quality suppliers offer it as a 98%+ pure product, complete with batch-specific certificates of analysis showing HPLC and NMR validation. Containers feature hazard pictograms required by GHS, with clear signal words and tightly defined storage instructions—detailing tightly sealed vials, away from moisture and direct sunlight. Lot numbers guarantee traceability for labs and factories. Labels state the IUPAC name, CAS number, purity, and net weight, along with emergency telephone numbers and QR code links to expanded safety data. This attention to detail doesn’t just support compliance—it creates a transparent record for audits and good manufacturing practice inspections.
Synthetically, the classic route begins with 3-methyl aniline, which reacts with chlorosulfonic acid to install the sulfonyl group at the ortho position. Cyclization under acidic conditions closes the thiazole, and controlled oxidation yields the final dione structure. Alternative approaches involve sulfonation of 3-methyl benzothiazole followed by oxidative rearrangement, sometimes using potassium permanganate or hydrogen peroxide as oxidants. Researchers fine-tune reaction times, temperatures, and solvent choices to optimize yield. Final purification often involves recrystallization from ethanol or acetonitrile and repeated washing to eliminate byproducts and residual acids.
The core dione structure attracts nucleophilic reagents, making substitutions at reactive positions feasible. Scientists often use it for condensation reactions, forming imines or hydrazones with primary amines and hydrazines. The methyl group at C3 activates ortho and para positions on the benzene portion, supporting further functionalization. Reductive modifications swap the dione for reduced thiazolidine rings, or halogenation at ring positions offers further diversification. Chemists appreciate the flexibility for creating custom structures, including fused-ring systems, dye precursors, and metal-binding ligands, each with their own performance or bioactivity profiles.
3-Methyl-1,2-benzothiazole-1,1-dione often appears in literature as 3-methylisothiazole dione, 3-methylbenzothiazole dioxide, or with trade names crafted by manufacturers. Researchers also encounter its identification numbers—CAS, EINECS, and regulatory catalog entries. Alternative notations pop up in patent filings and proprietary material data sheets. Product names sometimes reflect intended application, such as “Methylbenzothiadione Intermediate” or “BTD-3Me,” which lab teams adopt for shorthand. These variations in naming don’t just reflect marketing—they point to the compound’s role in broader chemical families and regulatory environments.
Safety data sheets mark this chemical as an irritant, with the potential for skin and eye irritation if handled without gloves or protective eyewear. Standard laboratory protocols call for chemical-resistant gloves, splash-proof goggles, and fume hoods. Dust formation can irritate respiratory passages, so weighing and transfers require attention to ventilation. For large-scale operations, closed system handling and local exhaust control systems lower worker risks. Shipping restricts the compound to well-sealed, labeled containers tracked by hazmat documentation. In accidental spills, dry inert absorbents work for cleanup, followed by careful decontamination of surfaces. Long-term storage calls for desiccators and controlled temperature rooms. These steps may seem involved, but they prevent exposure incidents and preserve chemical quality.
Chemists in dyes and pigments study this molecule for its strong chromophoric nature—offering unique hues and stability in color-fast applications, from textiles to advanced imaging agents. Pharmaceutical research teams consider it as a scaffold for anti-inflammatory, antimicrobial, and antiproliferative agents, drawn by its tunable reactivity and capacity for introducing diverse side chains. In materials science, it appears as a modifier of polymer backbones, lending thermal stability and electronic properties that fit the demands of sensors, OLEDs, or battery research. Synthetic organic labs deploy it in the discovery of novel heterocycles and ligands. Its adaptability fuels a wide reach across chemical industries and university research programs.
Research into new derivatives leverages high-throughput screening and combinatorial chemistry, rapidly producing libraries of analogs for drug discovery. Computational chemistry models predict binding modes for biological targets. Universities and corporate research groups publish papers on novel transformations—electrophilic aromatic substitutions, metal-catalyzed couplings, and ring expansions. Industrial labs focus on process intensification, scaling up reactions without sacrificing yield or purity. Collaborative research aims to link synthetic chemistry with pharmacology, toxicology, and material science, using interdisciplinary teams and shared databases for maximum impact and efficiency.
Toxicologists study the compound’s metabolic fate in animal models, measuring absorption, distribution, and elimination. Initial findings suggest moderate acute toxicity, largely driven by the dione’s electrophilicity and capacity for cellular disruption. Chronic exposure studies look for organ toxicity and genotoxic effects, with some concern over potential mutagenicity if metabolic breakdown exposes reactive intermediates. Bioassays show moderate environmental persistence, calling for careful wastewater management. Regulatory agencies review emerging data to set workplace exposure limits and clean-up requirements. Research continues, as teams update risk assessments with new findings, giving manufacturers and researchers updated guidance for safe and effective use.
Innovation teams are eager to unlock new reactivity patterns through careful tweaking of benzothiazole analogs. Automated synthesis and data-driven approaches promise access to hundreds of analogs for screening—not just in pharmaceuticals, but also as specialty materials and green chemistry reagents. Pushes to reduce environmental burden turn attention to degradation pathways and recyclable forms. Startups scan for underutilized application areas—medical diagnostics, agrochemical formulation, or advanced manufacturing—where this scaffold could offer a technical edge. As analytical and computational methods advance, deeper understanding of structure-activity relationships will keep 3-Methyl-1,2-Benzothiazole-1,1-Dione relevant in both discovery research and scalable production lines, echoing the way successful chemical intermediates shape multiple industries across the decades.
3-Methyl-1,2-Benzothiazole-1,1-Dione might not ring a bell for most people, but its impact rolls quietly through several industries. This synthetic molecule, also called methyl saccharin, does not sit on grocery shelves or pop up in drug commercials, but the work it does often ends up supporting products many people use every day.
Manufacturers use 3-Methyl-1,2-Benzothiazole-1,1-Dione as a chemical accelerator in the vulcanization process of rubber. Vulcanization makes rubber tougher, more resilient, and less sticky—qualities that keep tires from melting on hot asphalt or cracking in the cold. Chemical accelerators like this one help turn clunky, fragile latex into the rugged material used for tires, shoe soles, conveyor belts, and weatherproof seals.
From my own experience in automotive repair, the difference between untreated latex and fully vulcanized rubber jumps out immediately—a treated tire keeps its durability even after years on the road, handling thousands of miles and plenty of wear. That springy, almost stubborn resistance to damage comes down to what happens on the molecular level, thanks to chemicals like this.
Chemists rely on 3-Methyl-1,2-Benzothiazole-1,1-Dione as a building block for making other specialty chemicals. Specific lab syntheses use it to make dyes, pigments, and sometimes pharmaceutical intermediates. The compound's unique structure lets researchers craft more sophisticated or targeted molecules, giving rise to everything from advanced colorants in plastics or textiles to active ingredients in drugs.
Here's where things get interesting: much of the work done with this molecule takes place behind research lab doors. Innovations coming out of the chemical industry don't always make headlines, but a safer dye or a more effective drug starts with precise molecular engineering. Compounds like this often lead the way.
The big question—what about the safety of 3-Methyl-1,2-Benzothiazole-1,1-Dione? Regulatory bodies require careful handling and storage. Workers need protection, as skin and lung exposure can trigger irritation or worse. Strict rules require companies to keep emissions in check, and proper waste management keeps run-off away from local waterways.
During environmental audit work, I saw firsthand what happens when factories invest in proper filtration and disposal systems. It’s expensive up front, but fines and health lawsuits cost a lot more in the long run. Companies focusing on sustainability keep neighbors safer and earn trust.
Reliance on chemicals like 3-Methyl-1,2-Benzothiazole-1,1-Dione keeps modern industries moving, but it is not a free pass to ignore safety or community concerns. Supporting innovation means investing in greener chemistry, alternative synthesis methods, and transparency in supply chains.
The science keeps evolving, and demand for cleaner, less hazardous chemicals grows every year. By holding manufacturers accountable and encouraging new research, we all benefit from safer products without losing performance or quality.
3-Methyl-1,2-benzothiazole-1,1-dione might not roll off the tongue, but it finds a place in labs and factories where folks use it for making dyes and specialty chemicals. People who spend much time with this compound know it can irritate the skin, eyes, and lungs. Some dusts do more than make you sneeze; this one can actually burn or cause lasting damage. Accidental splashes or inhaling powder create real trouble. That’s why careful handling makes the difference between a routine workday and driving down to the ER.
Cotton T-shirts and jeans don’t cut it here. Folks need chemical-resistant gloves—like nitrile or neoprene, not cheap latex. Good goggles or a full-face shield protect eyes and skin from splashes. Work coats, aprons, and sleeves made for chemical work keep clothing and skin covered. Chemical dust doesn’t care about how careful someone feels. Real stories out of industry show that cuts and rips in gloves or sleeves leave the body wide open to burns or rashes. Replace damaged gear early, not late.
Open windows won’t handle the fumes or dust this chemical can give off. Real lab safety depends on working in a properly fitted chemical fume hood. If no hood sits nearby, don’t use it—a paper mask or bandana won’t block the dangerous bits. For anyone who worries about their lungs, get real with a respirator rated for organic chemicals and fit it well. In my early years working in a college lab, a classmate had to leave the room for fresh air after a small spill—no fun gasping for air, even briefly.
It’s tempting to tuck solid chemicals anywhere there’s room. Some people leave jars uncapped or near heat. 3-Methyl-1,2-benzothiazole-1,1-dione should stay cool, dry, and tightly sealed—preferably in a locked, labeled cabinet away from acids, bases, and anything flammable. Labels need to stay readable, using names and hazard info, not faded tape and bad handwriting. Don’t eat, drink, or store food nearby. I watched a janitor once clean up a chemical shelf—gloves off, coffee cup on the table—a recipe for accidental poisoning.
A working eyewash and safety shower belong in any room where folks handle chemicals like these. Showers and eyewash stations aren’t decorations for a lab tour; they save eyes and skin after a splash. Know the way to the nearest one, and check that it actually works. For spills, treat the chemical with a spill kit built for organics. Skip the paper towels—use absorbent pads, avoid breathing the dust, and always follow up by reporting any release, no matter how small it looks.
Reading labels means more when training covers what can actually happen with mistakes. Review safety data sheets before using anything, not after finding red eyes and shortness of breath. Ask questions, especially when new to the chemical or the building. Regular training refreshers, even short safety drills, help everyone remember why these rules matter. Trust in protocols and good habits, not luck.
From storage to cleanup, each task around 3-Methyl-1,2-benzothiazole-1,1-dione deserves care and solid habits. Most labs count on teamwork—watching out for spills, reporting near misses, and checking each other’s safety gear. With some chemicals, experience tells you, “I wish I’d put on that extra glove.” Better to hear that voice sooner—before a mistake lands you in the hospital.
Anyone who spends time around chemical storage quickly learns the importance of respect—respect for what a substance can do, both good and bad. 3-Methyl-1,2-Benzothiazole-1,1-Dione, often found in research and synthesis labs, calls for that kind of clear-eyed approach. Based on my own lab days, a little vigilance has saved more than a few headaches—and cleared up noxious fumes before they got out of control.
In older labs, labeling faded and caution sometimes slipped. I’ve watched improperly stored containers trigger confusion and even some mild panic when leaks or odd smells crept into hallways. This kind of compound, with its potential for irritation and reactivity, won’t give second chances. That means hats off to routine checks, clear labeling, and an understanding of risks before a crisis starts brewing.
Humidity and heat change the game in chemical storage. High temperatures may nudge a compound toward decomposition or changed activity. I recall a storage room hit by a broken AC unit—honest mistake, but some containers bloated and crumpled under the heat. For 3-Methyl-1,2-Benzothiazole-1,1-Dione, a cool, dry place in a well-ventilated, designated cabinet lowers the risk of breakdown or dangerous fumes. Running a climate log never hurts; jotting down readings has caught more than one quietly creeping temperature spike before damage happened.
There’s a simple rule that’s easy to ignore in a busy work environment: segregate chemicals based on type and hazard class. I watched a friend learn this the hard way when cross-contamination ruined an entire batch—not to mention the cleanup. Strong acids, bases, and reactive metals shouldn’t share a shelf with organosulfur compounds. Corrosion and reactivity both tend to rear their heads when mixed. Using secondary containment—like plastic bins—adds a physical barrier and can keep a minor leak from turning into a whole-room emergency. This approach echoes the wisdom of seasoned safety officers who’ve been around enough spills to treat every chemical as a potential wildcard.
Part of the confusion I’ve seen stems from sloppy, handwritten labels or missing hazard details. That’s a gamble nobody should take. Every bottle deserves a label with the substance name, date received, and hazard info. This ritual is as basic as handwashing in a pandemic. Even a tired grad student can glance and understand what’s at stake or how to respond in an accident. Back in the day, a quick-reacting team avoided major trouble because someone caught a hazard icon in a split second—details save skin and peace of mind.
Personal protective equipment like gloves, goggles, and masks don’t replace careful handling or tidy storage. They add a layer of trust but won’t undo mistakes once they snowball. I remember a story of a shared workspace where one careless move sent fumes up through the air vents, triggering alarms and sending half a building outside. Since then, I always pay closer attention to both the shelf and the workspace; both deserve the same respect.
Small actions—routine checks, controlled storage conditions, clear barriers, and sensible labels—do a lot more good than waiting for an emergency protocol. Based on plenty of sweaty afternoons and too many close calls, it’s easier to act with intention up front. Those few minutes organizing a shelf can spare days dealing with paperwork, lost samples, or worse, injury. That’s an investment worth making in any lab, big or small.
Ask anyone who works with chemicals—structure always changes the game. Shape, arrangement, placement of every atom makes a world of difference in how something interacts with its surroundings. That’s what got my curiosity going with 3-Methyl-1,2-Benzothiazole-1,1-Dione. Some people call it a sibling to isatoic anhydride, yet the “methyl” tag and benzothiazole backbone mean it takes another path in reactivity and uses.
Chemists know every atom placement counts. In this case, you start with a benzothiazole ring—a fused ring made by benzene and thiazole working together. Now, throw in a methyl group at position three, and look at the “1,1-dione” part: two double-bonded oxygens anchored to the nitrogen at position one. Scratching this out on paper only tells so much. Try building it with a kit or sketching on a napkin, and it jumps out—this isn’t some simple aromatic compound anymore.
Its chemical formula clocks in at C8H7NO2S. The methyl group (–CH3) hugs the third carbon on the ring, shaping both electronic effects and stability. Those double-bonded oxygens don’t just hang around either. They dial up reactivity, marking this molecule for modified behavior in reactions. It’s less forgiving than classic anhydrides, more reactive than many thiazole derivatives, and packs a punch in every synthesis lab that’s ever tried to make something from it.
Some might see it as another compound to catalog, but anyone handling even one gram must respect that molecular structure. The two carbonyls on nitrogen set off alarms in the brain: they drive hydrolysis super fast and steer all sorts of reactions you’d want or want to avoid. Synthesis isn’t smooth sailing either. Paths to 3-methyl-1,2-benzothiazole-1,1-dione usually demand careful planning—most start from methyl-substituted anilines or thioamides. These routes need clean conditions, sharp attention to byproduct formation, and plenty of ventilation, not just because of fumes but because side-reactions ramp up when you’re working with such a reactive framework.
Labs see its potential: precursor to dyes, biomedical agents, sometimes even agrochemicals, thanks to the unique push-pull of the thiazole and the two carbonyls. Every use brings a flip-side. Any process that plays with sulfur and nitrogen—especially wrapped in a benzene core—warrants respect. Spill a thiazole derivative in a lab, and you’ll understand fast why structure matters for safety: reactivity climbs, handling changes, and waste disposal turns complicated.
The structure pins down how it travels through the environment, too. Benzothiazole rings show up in rubber industry runoff, fueling debate about toxic breakdown products. Every feature of its structure—aromaticity, sulfur, dual carbonyls—steers its movement, persistence, and potential harm. Most chemical databases and regulators urge caution, and safety sheets won’t let you forget—correct labeling, controlled storage, and smart disposal keep both people and environment safer.
Benzothiazole diones like this one aren’t going away. Safer synthesis approaches make a difference. Look to greener oxidants, fewer hazardous solvents, or new catalysis strategies. Tighter rules and informed chemists can limit pollution, while detailed databases (like PubChem and ChemSpider) give a blueprint for understanding every bump, bend, and reactive site. Structure isn’t just an image in a textbook; it’s the reason a molecule matters—and how we handle it shapes our world.
Breathing or touching chemicals at work isn’t always as simple as following instructions. 3-Methyl-1,2-Benzothiazole-1,1-Dione—often called N-Methyl Saccharin—has started to draw fresh attention in workplace safety circles. The structure, containing both sulfur and nitrogen rings, makes chemists appreciate how it brings unique properties to drugs, dyes, and rubber. Most folks working in labs or on manufacturing lines glance at the label and only see a pile of letters.
Living with chronic asthma myself, any mention of chemical dust makes my skin crawl. Many aromatic compounds carry sneaky risks—just because something smells sweet or isn’t listed as toxic doesn’t mean it’s safe to inhale or touch. Occupational Safety and Health Administration listings warn against skin and eye contact with benzothiazoles. There’s no reason to think this methylated version behaves any safer. Several related compounds irritate mucous membranes, trigger coughing, and hurt the lungs over time. The science suggests repeated low-level exposure isn’t as harmless as companies sometimes want to believe.
Wastewater streams from factories can send this molecule right into local rivers without proper management. It doesn’t break down in sunlight or water quickly, which means it lingers in the environment. Over years, things add up. Fish and aquatic creatures soak up these molecules, which then end up in food chains. It’s a pattern seen before with polycyclic or sulfur-containing chemicals: small leaks or improper dumping snowball into ecosystem threats.
Recent case studies from China and Western Europe show that environmental monitoring sometimes lags behind industrial innovation. Trace levels of benzothiazole derivatives have popped up in drinking water, especially near tire-recycling plants and dye factories. It's tempting to dismiss parts-per-billion as trivial, but the science on endocrine disruption and cancer risk keeps evolving. It reminds me of conversations about BPA or phthalates years ago—society often figures out these dangers much too late.
Wearing gloves, eye protection, and dust masks protects workers from acute skin and lung irritation. Fume hoods and well-ventilated work rooms create safer breathing spaces. Training employees to recognize chemical names and understand how adding a methyl group to a molecule can change its toxicity gives everyone more control when trouble arises on the job.
Good housekeeping matters. Keeping storage tightly sealed, labeling everything clearly, and cleaning spills right away leaves less room for mistakes. For companies, investing in industrial scrubbers and state-of-the-art water treatment keeps pollutants from slipping through. Environmental audits and community engagement back up promises with proof. If regulators step in with tighter discharge limits or new hazard testing, businesses can adapt faster with these measures already in place.
Ignoring warning signs cost lives and wrecked environments in the past, and the pattern shouldn’t repeat. Scientists owe it to society to keep digging into the long-term effects of new chemicals, not just focusing on short-term profits. Neighbors near plants, workers on factory floors, and local wildlife all deserve the extra caution. Respect for science, transparent reporting, and a willingness to fix gaps set the bar higher for everyone. Real safety comes from facing potential risks honestly and acting before the headlines start rolling in.
| Names | |
| Preferred IUPAC name | 3-Methyl-1,2-benzothiazole 1,1-dioxide |
| Other names |
3-Methyl-1,2-benzisothiazole-1,1-dioxide 3-Methylsaccharin |
| Pronunciation | /θriː-ˈmɛθɪl-waɪ.tuː-bɛnˈzoʊ.θaɪ.əˌzoʊl-waɪ.wʌn.daɪˈoʊn/ |
| Identifiers | |
| CAS Number | 723-36-6 |
| 3D model (JSmol) | `3d:JSmol?cid=179279` |
| Beilstein Reference | 158566 |
| ChEBI | CHEBI:87673 |
| ChEMBL | CHEMBL410243 |
| ChemSpider | 24634 |
| DrugBank | DB08438 |
| ECHA InfoCard | 01-2119977695-20-XXXX |
| EC Number | 401-720-1 |
| Gmelin Reference | 9889 |
| KEGG | C14126 |
| MeSH | D010578 |
| PubChem CID | 156601 |
| RTECS number | DC3325000 |
| UNII | B90GM90M9F |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C8H7NO2S |
| Molar mass | 193.22 g/mol |
| Appearance | White to off-white solid |
| Odor | Unpleasant |
| Density | 1.41 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 1.24 |
| Vapor pressure | 0.0563 mmHg (25°C) |
| Acidity (pKa) | 1.60 |
| Basicity (pKb) | 8.46 |
| Magnetic susceptibility (χ) | -61.2 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.677 |
| Viscosity | 76.0 cP (20°C) |
| Dipole moment | 3.25 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 239.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | –47.4 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -4499.8 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P333+P313, P337+P313, P362+P364, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | > 196.6°C |
| Lethal dose or concentration | LD50 (oral, mouse): 640 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat 670 mg/kg |
| NIOSH | RN6471 |
| PEL (Permissible) | Not established |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Benzothiazole 1,2-Benzothiazole-1,1-dione 6-Methyl-1,2-benzothiazole-1,1-dione 2-Methylbenzothiazole 3-Methylbenzothiazole |