The roots of 3-Methoxythiophene reach back to early experiments with heterocyclic chemistry in the mid-20th century. Chemists drawn by the challenge of understanding and modifying sulfur-containing rings soon saw the value in tweaking the parent thiophene molecule. Adding a methoxy group onto the structure brought fresh reactivity and handling differences, compared to plain thiophene. Over decades, work in academic labs and later industrial settings centered on mild functionalization and the search for reliable production lines. Techniques moved from batch to continuous-flow, often driven by demands from the electronics sector and from synthetic chemists chasing new scaffolds for pharmaceuticals.
3-Methoxythiophene pops up in catalogs for fine chemicals, standing out thanks to its clear, pale-yellow look and strong, characteristic odor. Most chemists recognize it from its role in making more complex compounds, especially in the fields that care about the electron-rich properties of thiophenes and the handy placement of the methoxy. Demand comes from universities running organic classrooms, but plenty lands in start-ups and industrial outfits looking to find the next polymer or small molecule with standout properties. A molecule like this draws orders not because it is rare but because its reputation for reliability and consistent reactivity matters in complicated syntheses.
The colorless liquid nature of 3-Methoxythiophene, with a boiling point floating around 148-150°C and a melting point that stays far below room temperature, gives it utility and some danger — with volatility making storage and handling more than just a matter of screwing a cap tight. The density hovers near 1.09 g/cm³, enough that even a small bottle stays heavier than most would guess. Its solubility in organic solvents, from ether to acetone, means it plays well with modern reaction setups, but water solubility remains low. Its chemistry starts from a foundation of a five-membered sulfur ring, with the methoxy group on position 3 swinging its electron-donating influence across the pi system, tuning its reactivity for both nucleophilic and electrophilic reactions.
Catalog entries and supply sheets always mention purity. The industry standard means at least 98% purity, with GC or NMR traces put in as proof. Labels call out the CAS number 1559-91-9 and show hazard pictograms warning of both flammability and risks if inhaled. Typical storage recommendations ask for temperature control, dark bottles, and snug seals to tame evaporation. Even so, periodic tests catch leaky seals, and backup storage in dedicated flammables cabinets brings peace of mind. The Safety Data Sheet spells out thresholds for workplace exposure, relating to both short-term vapor contact and longer-term risks.
Most modern synthesis routes rely on O-alkylation of 3-hydroxythiophene. In practice, this often means reacting 3-hydroxythiophene with methyl iodide or dimethyl sulfate under weakly basic conditions, such as potassium carbonate in a solvent like dimethylformamide or acetone. This pathway tends to be both clean and scalable, giving a decent yield without flooding the mix with side products. There's also a route involving catalytic methylation using dimethyl carbonate, sought after by those aiming for greener chemistry and safer waste streams. Old patents describe chloromethylation of thiophene followed by substitution with methoxide, but few choose this route given the more modern, less hazardous alternatives.
On the bench, 3-Methoxythiophene acts as a launching pad for a handful of key transformations. Direct coupling reactions, such as Suzuki or Stille cross-couplings, link the thiophene ring with aryl or alkenyl partners. The methoxy group can withstand many milder conditions, allowing functionalization at the 2- and 5-positions. Electrophilic substitution occurs faster at these free positions, making halogenation or Friedel-Crafts acylation straightforward. Researchers use this backbone to build more elaborate structures, including fused rings or introducing nitro groups for further downstream chemistry. Sometimes, partial reduction or oxidation lets chemists tune the electron distribution, changing the compound’s use for electronics versus pharmaceutical scaffolding.
Beyond its IUPAC name, you’ll spot 3-Methoxythiophene listed as 3-Methoxy-thiophene, m-Methoxythiophene, or by the simple abbreviation 3-MeOT. Some catalogs just stick with Methoxythiophene, though this brings a risk of confusion with its 2-substituted isomer. Trade names rarely diverge from these basics because regulatory filings and literature tend to converge on the original IUPAC conventions for registration and tracking. In research articles and patent filings, variations appear, but smart practice means spelling out the structure on first mention to avoid error.
Anyone handling 3-Methoxythiophene needs to respect its volatility and flammability. Fume hood use is non-negotiable, given both the unpleasant odor and mild toxicity on inhalation. Even with gloves and eye protection, the compound’s volatility can catch workers off-guard if left open too long or when decanting larger volumes. Spills spread quickly and cleaning up leftover vapors remains tricky without good airflow. In larger-scale syntheses, automated delivery systems cut down on operator exposure, and many labs have invested in air monitoring. Emergency showers and eyewash stations become essential, especially since the compound can irritate skin and eyes. Spill kits with absorbent pads and specific neutralizers turn what could be a panic into a manageable event, and staff drills build routine in safe handling. Fire regulations classify it as a Class 3 flammable liquid, so fire suppression tools and evacuation training come standard in organizations that use it often.
Research into conductive polymers leaned on 3-Methoxythiophene for building blocks that offered solubility and electronic tunability. In organic electronics, its presence gives polymers both flexibility and resistance to decomposition. The pharmaceutical world uses the compound as an intermediate in routes to potential drug molecules, particularly in scaffolds designed to mimic or block biological activity. Some agrochemical development teams also check its usefulness for tuning bioactivity in new crop protectants. Beyond this, specialty dyes and organic synthesis textbooks highlight it for teaching and exploration. For newcomers to the lab, running reactions with 3-Methoxythiophene ends up becoming a memorable introduction to handling reactive and smelly, but not extremely hazardous, chemicals.
Academic groups and R&D teams push the boundaries by exploring new functionalization strategies and coupling techniques. The focus often falls on green chemistry, with efforts to replace traditional reagents with less toxic or recyclable alternatives. Investigation into direct C-H functionalization without metal catalysts seeks to bring both economic and sustainability benefits. Collaborations with electronics companies have produced improved organic semiconductors and photovoltaic materials, using the 3-methoxythiophene unit to enhance performance. Pharmaceutical research, always eager for fresh heterocycles, treats this molecule as a launching point for analogs with improved solubility or target affinity. Secure supply chains and on-site synthesis protocols reflect the growing demand, ensuring the molecule meets rigorous reproducibility and purity standards required for cutting-edge research.
Toxicological studies remain somewhat limited compared to more widely used benzene derivatives. Animal studies show low to moderate acute toxicity, with inhalation leading to headaches, dizziness, and irritation at higher doses. Chronic exposure studies suggest moderate organ impacts, but most data stems from high exposure and not typical laboratory or industrial use. Environmental fate studies indicate that while 3-Methoxythiophene does not bioaccumulate, its breakdown in water and soil produces smaller molecules, most of which are less toxic but must still be tracked. Regulatory agencies in North America and Europe recognize its hazards primarily in manufacturing and handling, rather than from consumer exposure. Safety data underscore the importance of minimizing releases and ensuring good ventilation, as repeated minor exposures could lead to cumulative effects over time.
Looking ahead, 3-Methoxythiophene stands out as a key target for greener synthesis and sustainable process development. Interest from electronics, optoelectronics, and polymer science continues to grow, especially among researchers eager for organic materials that outperform traditional silicon-based options. Improvements in catalytic functionalization and process intensification hint at more efficient routes, lower waste, and safer working environments. Some industry insiders expect the next generation of organic light-emitting diode (OLED) displays and photovoltaic panels to depend on new derivatives built from this molecule’s backbone. Meanwhile, regulatory shifts push for reduced solvent waste and tighter emission controls, prompting manufacturers to update both process and product stewardship protocols. Academia keeps investigating its effects and modifications, ensuring that fresh applications will emerge in the years to come as both analytical techniques and creative synthetic pathways develop.
3-Methoxythiophene sits in the world of specialty chemicals, probably unfamiliar to most folks outside chemistry or electronics. I once walked through a university lab full of pungent smells and small glass vials, where the chemists explained how these little compounds pave the way for high-tech breakthroughs. Despite its humble appearance, this molecule powers several major industries, mainly because it brings special qualities to the table.
Anybody who has fiddled with old gadgets knows that electronic components keep shrinking and getting more flexible. 3-Methoxythiophene shows up in research and manufacturing of organic electronics. Its structure allows scientists to build conductive polymers, a core ingredient in flexible solar cells, touchscreens, and organic light-emitting diodes (OLEDs). From reading up on progress in materials science, it’s clear these new plastics aren’t just for show. They cut down on weight, open up new form factors for wearable tech, and help bring down manufacturing costs.
A handful of years ago, this kind of technology felt out of reach even for labs, let alone everyday homes. Now, through clever chemistry involving compounds like 3-Methoxythiophene, flexible displays and solar panels head toward mass-market devices. As more countries chase clean energy, the hunger for materials that build affordable, reliable organic solar cells has never been greater.
Most pipelines for new drugs blend natural and synthetic chemistry. In drug design, scientists want to introduce sulfur atoms in very precise ways, which isn’t simple. Thiophenes, including 3-Methoxythiophene, offer a place to start. They can help researchers attach new side chains or develop active ingredients that the body absorbs more efficiently. Reading industry reports, I’ve learned that sales of complex molecules have grown steadily. The pharmaceutical field always looks for new ways to improve bioavailability and reduce side effects, so ingredients like this play a quiet, vital role.
The plastics industry isn’t just about bottles and bags anymore. Tomorrow’s cars, planes, and buildings need materials that stay strong, flexible, and conduct electricity when asked. 3-Methoxythiophene can join polymer chains, opening the door for antistatic coatings or special conductive fibers. Developers look for ways to replace heavy metals with greener options, and this sulfur-based compound is part of the solution. A material with this kind of sulfur-carbon ring can change everything from how tennis rackets perform to how smart packaging talks to store shelves.
Using volatile building blocks like 3-Methoxythiophene demands strict safety and expertise. Workers need solid training in lab environments, with careful handling under stringent ventilation. The chemical’s odor and reactivity have led to specialized storage protocols in almost every industrial setting where it appears. Accidents can have serious health effects, so access remains limited to pros in the field.
Manufacturers are learning to track the life cycle of chemicals like 3-Methoxythiophene, from creation to disposal. The push for cleaner production keeps growing. For companies involved in electronics, drug manufacturing, or advanced plastics, green chemistry aims to cut hazardous byproducts without losing performance. That means investments in both research and training keep rising. People who care about both innovation and safety are likely to find new ways to put specialty sulfur compounds to work without increasing risks.
3-Methoxythiophene isn’t a household name, yet its impact shapes products we’ll depend on in future homes, hospitals, and everyday lives. None of this happens without careful oversight, skilled staff, and smart design—three things that never go out of style.
3-Methoxythiophene belongs to the family of thiophene derivatives, and these compounds often pop up in conversations around modern organic chemistry labs. As someone who’s spent plenty of hours tucked away in chemical storerooms, I can vouch for the subtle power a small adjustment like adding a methoxy group at the 3-position brings. Right away, let’s spell out its formula: C5H6OS. That’s five carbon atoms, six hydrogens, one oxygen, and a sulfur atom. No extra pieces or tricky components hiding under longer names.
Walk into any lab where the synthesis of heterocyclic compounds is underway, and you’ll spot bottles marked “3-Methoxythiophene” on a shelf. The molecular weight isn’t just a number for a chemist; it’s a basic check before preparing solutions or figuring out reaction yields. For this molecule, the calculation is pretty straightforward. Carbon clocks in at about 12.01 g/mol, hydrogen adds up around 1.01 g/mol, oxygen weighs 16.00 g/mol, and sulfur brings 32.07 g/mol to the mix. Add these up according to the formula:
Total those numbers and you land at 114.18 g/mol. Knowing this isn’t trivia — it influences everything from predicting how much to weigh out for a reaction to calculating the precise amounts needed for scale-up in industry applications.
Some folks might skim over these facts, thinking they’re just technical details. Yet when you’ve had to troubleshoot a sticky chromatography issue or struggled to get a precise melting point at the bench, you realize these details are part of a bigger picture. Misjudging molecular weight leads to wasted reagents or miscalculated product yields. This isn’t just about numbers; it’s about trust in your process and getting reliable results.
The significance of 3-Methoxythiophene doesn’t end at molecular structure or numbers. Thiophene derivatives play a big part in organic electronics and pharmaceuticals. In synthetic routes, every atom counts for both purity and safety. I remember seeing quality assurance teams pull entire batches because just a sliver of the wrong isomer crept in, knocking molecular weight and test readings off track. Building deep familiarity with these core properties helps avoid that headache.
Information about basic properties like chemical formula and molecular weight needs to reach not just the researcher, but students, technicians, and procurement managers too. Training that highlights the rationale behind these details — not just rote memorization — builds better judgment in the lab. Digital systems that cross-check chemical data against up-to-date databases reduce the risk of costly manual errors. Even simple posters near balances or storage cabinets make a difference, anchoring these key details in daily routines.
People outside organic chemistry circles might not get excited about 3-Methoxythiophene, but it’s hard to overstate its quiet importance. From calculating safe handling, patenting a new electronic material, or teaching the next crop of scientists, it’s these precise details that ground good research and sound manufacturing choices.
Whenever you reach for that bottle labeled 3-Methoxythiophene, keep in mind: chemical formula C5H6OS and molecular weight 114.18 g/mol form the backbone of its real-world usefulness. It’s these pieces of applied knowledge that keep science moving forward — in classrooms, labs, and large-scale production alike.
3-Methoxythiophene sits on many lab benches as a useful building block for pharmaceutical and electronic applications. Its popularity comes from a combination of its reactive nature and strong, sometimes overpowering, odor. That kind of pungent smell isn’t just unpleasant—it offers a warning. My time in lab settings taught me to respect chemicals with noticeable aromas because they often mean business when mishandled.
A bottle of 3-Methoxythiophene takes just fine to cool, dark storage. The best bet lies in a flammables cabinet, one kept away from heat sources or direct sunlight. Flammable chemicals keep their temperament under control better at lower temperatures, so aim for a temperature out of the heat range—room temperature can work, but some prefer cold rooms for extra safety. Humidity can be an issue; keep things dry and tightly sealed. Corrosion-resistant containers help, and if glass is used, swapping out faulty stoppers quickly prevents nasty leaks.
A hood, not a regular bench, is the best place to open a bottle. Even a quick whiff at the stopper delivers a hit that lingers in the sinuses for hours. Using proper PPE—nitrile gloves, goggles, and a lab coat—never feels excessive here. I learned in a shared facility that spills of 3-Methoxythiophene draw a crowd, since it doesn’t just disappear; it drifts. Good ventilation keeps the headache at bay.
This compound stays flammable enough to turn a spark into a fireball if handled near open flames or static. Grounding containers and avoiding wool sweaters on dry days help lower that ignition risk. Inhalation, even in low amounts, dries out mucous membranes and irritates airways. Anyone with asthma or allergies notices the impact faster. Direct skin contact can trigger irritation or, in rare cases, an allergic response. Cleanup requires more than a paper towel—absorbent spill pads and proper waste segregation matter much more than a quick mop.
Labeling isn’t optional. It clears up confusion and prevents people from opening something by mistake. Mixing this chemical with strong oxidizers or acids ramps up the risk of violent reactions. So, store separately, never on the same shelf. I remember a case in grad school where a careless shelf assignment almost meant disaster—a reminder that shared knowledge and clear rules keep everyone safe.
Plan ahead for disposal. Most facilities require spent or surplus material to go into designated hazardous waste bins. Pouring leftovers down the sink or tossing them in regular trash isn’t just reckless—it’s illegal in most jurisdictions. Waste services often provide specific bins for volatile organics, and regular pickups keep accumulations down. These measures shield staff, the environment, and downstream workers from exposure.
Even experienced researchers forget the basics sometimes. Regular refresher training helps cement habits. Signs posted in storage rooms offer quick reminders: keep containers shut, check the expiration or degradation, and report drips or leaks. Having a chemical spill kit stocked and ready means accidents turn into inconveniences, not emergencies. The same simple steps fit both the professional lab and the academic chemistry department.
Anyone handling 3-Methoxythiophene owes colleagues, themselves, and the future a commitment to respect its hazards. Safe storage and good habits give everyone more time to focus on innovation—not injuries or cleanup. That’s how real lab progress gets made safely.
3-Methoxythiophene doesn’t exactly jump off the shelf at most stores, but it pops up often in laboratories, synthetic chemistry, and sometimes in electronics research. It’s a colorless liquid, used mainly as a building block in organic synthesis. The folks working in labs will recognize its distinctive smell—sort of sharp and, honestly, not very pleasant. The safety data behind this chemical isn’t buried in jargon. It's an industrial compound, not something to splash around without care.
Looking at what science says, 3-Methoxythiophene is hazardous in a few well-defined ways. First, the vapors irritate the eyes and respiratory tract. If you’ve ever worked with compounds that sting the nose or make the eyes water, you’ll recognize it. Skin contact also brings trouble. It can trigger allergic reactions or serious itching if spilled. I’ve read about cases where a person got careless and wound up with irritation that lingered for days.
Short-term exposure in high concentrations can lead to symptoms most people consider mild—coughing, headaches, dizziness—but the risks stack up with poor protection or improper ventilation. Ingesting it or consistently breathing the vapors takes a toll on organs over time. Animals exposed to high amounts have shown toxic effects on the liver and kidneys, though there’s not enough data yet to say for sure exactly what the threshold is for humans.
Regulatory databases, like those maintained by the European Chemicals Agency (ECHA) and the U.S. National Library of Medicine’s PubChem, list the substance as hazardous. It's also marked with the Health Hazard symbol under the Globally Harmonized System (GHS). That means there are real concerns about its impact if handled carelessly. While you probably won’t see mass poisonings, individual exposure can add up, especially among workers who treat this compound as benign.
Lab safety stories pass through every cohort of students—rushed cleanups lead to skin burns, sniffing fumes to “check purity” results in long afternoons with headaches. I remember one enthusiast who cut corners on glove changes during organic synthesis, only to develop a nasty rash that left him out of the lab for a week. Chemicals like 3-Methoxythiophene reinforce those lessons.
Proper house ventilation, fume hoods, and gloves matter. Not as a faint recommendation, but as real safeguards. I’ve learned that trusting your senses is not good enough; nose blindness sets in fast, and vapors creep without sound or warning. Safety engineers and responsible supervisors check the ventilation and ensure that anyone working with this compound has access to Material Safety Data Sheets and knows how to use spill kits and eye-wash stations.
Education stays critical—everyone working in a lab should recognize the signs of chemical exposure and take them seriously. Using personal protective equipment, enforcing good cleanup habits, and keeping work areas organized keeps small accidents from spiraling into dangerous situations. Preferring closed-system processes that limit airborne release reduces exposure risks. I’ve seen labs review incidents and adjust procedures so the same mistakes don’t happen twice.
Ultimately, respect for chemicals like 3-Methoxythiophene makes a safer workspace. This isn’t about overreacting to every bottle in the storeroom, but about valuing health over convenience. Workers, students, and supervisors stay safest by keeping up with the facts, recognizing hazards, and not taking shortcuts with things you’d never want near your coffee mug or bare hands.
3-Methoxythiophene plays a vital role in various synthesis routes, especially when developing pharmaceuticals, manufacturing advanced polymers, or pushing innovation in electronics. Organic chemists know this compound as a convenient building block. It features a thiophene ring substituted with a methoxy group, and small tweaks like this often lead to big changes in the final properties of advanced materials. Purity and packaging options both matter quite a bit; they shape everything from lab workflow to final application reliability.
High purity always drives better experimental repeatability. For 3-methoxythiophene, suppliers often provide material with purity no less than 98%, as verified by GC or HPLC. A low water content is critical, with certain high-grade batches containing less than 0.5% water or sometimes even less than 0.2%. Reputable chemical manufacturers share a certificate of analysis, listing precise purity plus breakdown of common impurities (methylthiophenes, sulfur oxides, and others). Researchers who care about sensitive electronics or catalytic chemistry especially pay attention, since trace impurities can poison catalysts or skew measurements by many percent. From my time running small organic reactions, I can vouch that you simply don’t get the same results using off-spec solvent, and a little impurity always finds a way to show up in NMR or mass spec.
Pack size brings its own set of choices. Most chemical vendors offer small laboratory bottles, with 1-gram or 5-gram vials making sense for pilot studies and early research. At the next level, 25-gram or 100-gram bottles supply larger academic labs or small industrial test runs. Scale-up for manufacturing often means shifting to 500-gram or 1-kilogram bottles, sometimes presented in amber glass for light-sensitive chemicals like thiophenes. Handling larger sizes also brings safety and cost considerations—3-methoxythiophene arrives as a liquid with a distinct odor, and many labs lean on special solvents cabinets and PPE. Bigger factories sometimes negotiate for custom drum sizes or returnable containers to cut waste and logistics costs.
Glass bottles remain the standard for most 3-methoxythiophene shipments, since they resist the solvent action of many organosulfur chemicals. Sometimes fluoropolymer-lined containers step in, especially for sensitive or long-term storage. I’ve had shipments that turned up with leaking plastic—never again. Seals matter, too. PTFE-lined caps or crimp seals cut down air and moisture ingress, which helps maintain purity for longer.
Trust in a chemical source flows from transparency. I always prefer a manufacturer that openly posts analytical data, batch numbers, and real contact information. The chemical supply world isn’t immune to counterfeit products, especially for rare heterocycles. Many scientists lean on established networks and peer-reviewed purchasing recommendations as insurance against products that fall short. GMP labeling enters the picture for pharmaceutically focused labs, where process documentation and contamination control aren’t just nice-to-haves—they’re the law.
It pays to treat any new bottle as a potential hazard until proven otherwise with a quick GC check or NMR test. Setting up clear labeling and handling protocols reduces cross-contamination and makes reorder decisions easier. If workplace injuries linked to chemical mishandling still happen, it’s often due to ignored storage or repackaging rules. Industry can do more by designing smarter caps, tamper-evident bottles, and cleaner return-loop drums. Every step up in purity or responsible packaging raises the bar for both safety and scientific rigor.
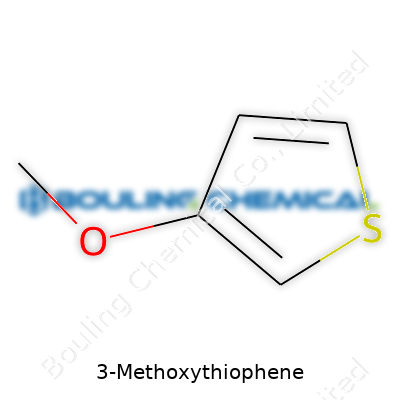
| Names | |
| Preferred IUPAC name | 3-Methoxythiophene |
| Other names |
3-Methoxy-1-thiophene 3-Methylthiofuran 3-Methoxythiophen |
| Pronunciation | /ˈθaɪ.oʊ.fin/ |
| Identifiers | |
| CAS Number | 829-98-5 |
| Beilstein Reference | 1598734 |
| ChEBI | CHEBI:83439 |
| ChEMBL | CHEMBL147631 |
| ChemSpider | 154180 |
| DrugBank | DB03780 |
| ECHA InfoCard | ECHA InfoCard: 100.013.883 |
| EC Number | 2465-27-2 |
| Gmelin Reference | Gmelin Reference: 107404 |
| KEGG | C06135 |
| MeSH | D017887 |
| PubChem CID | 11757 |
| RTECS number | KL8925000 |
| UNII | B5B4WSY53A |
| UN number | UN3439 |
| CompTox Dashboard (EPA) | DTXSID2020325 |
| Properties | |
| Chemical formula | C5H6OS |
| Molar mass | 114.16 g/mol |
| Appearance | Colorless to yellow liquid |
| Odor | aromatic |
| Density | 1.116 g/mL at 25 °C |
| Solubility in water | slightly soluble |
| log P | 1.68 |
| Vapor pressure | 0.689 mmHg (at 25 °C) |
| Acidity (pKa) | 6.60 |
| Basicity (pKb) | 7.80 |
| Magnetic susceptibility (χ) | -53.2·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.551 |
| Viscosity | Viscosity: 0.782 cP (20°C) |
| Dipole moment | 1.43 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 177.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -26.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3738.4 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H315, H319, H335 |
| Precautionary statements | P210, P280, P305+P351+P338, P337+P313 |
| Flash point | 49 °C (120 °F; 322 K) |
| Autoignition temperature | 215 °C |
| Explosive limits | Explosive limits: 1.3% - 7.6% |
| Lethal dose or concentration | LD50 (oral, rat): 820 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 990 mg/kg |
| NIOSH | GNF59710 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5 – 10 |
| Related compounds | |
| Related compounds |
Thiophene 2-Methylthiophene 3-Methylthiophene 2-Methoxythiophene |