Looking back, the roots of 3-Ketomorpholine reach deep into the progress of synthetic organic chemistry in the twentieth century. The early chapters involved chemists playing with morpholine’s basic ring, driven by curiosity and the need for new building blocks in both pharmaceutical and chemical manufacturing. Interest in N-heterocycles picked up as researchers realized how central morpholine derivatives could be for drug scaffolds and for tough intermediates in specialty chemicals. Over decades, tweaking the oxygen and nitrogen positions, adding or shifting ketone groups, and refining purification trickled into refining access to 3-Ketomorpholine. The substance waited in obscurity for a while, overshadowed by its cousin morpholine, yet steadily developed a reputation among drug innovators and material scientists who needed reliable, repeatable synthetic routes.
3-Ketomorpholine stands as a classic example of a small target compound with outsized utility for chemists. The structure grafts a carbonyl group right onto the morpholine ring, setting the stage for all sorts of modifications and further reactions. On the shelf, the chemical shows up as a colorless liquid or sometimes as off-white crystals, with a distinct odor that reminds you quickly this is no table sugar. More than a lab oddity, the compound finds its way into research labs, process scale-ups, and in industrial pipelines where researchers and engineers need a reactive intermediate—something chippy enough to build on, but stable enough to hold up under routine storage.
If someone picks up a vial of 3-Ketomorpholine, they’ll notice its low viscosity—almost like water—thanks to the small size and low molecular weight. The boiling point usually lands around 180–200°C, giving it enough thermal stability to handle most heated reactions without breaking apart. The melting point usually hovers near 25°C, so the compound might sit as a solid or a slightly oily liquid at room temperature, depending on ambient conditions and purity. Its solubility is broad, dissolving cleanly in water, ethanol, and many polar organic solvents, making life easier when it comes to formulation or reaction prep. The functional groups line up: the oxygen and nitrogen in the ring both carry lone pairs, and the central ketone oxygen pulls in nucleophiles, adding intrigue to its chemistry. In the lab, it’s not overly volatile, but still deserves respect—avoid inhaling fumes or letting liquid pool on skin.
Manufacturers provide 3-Ketomorpholine under strict spec ranges for industrial and R&D use. Purity runs at or above 98%, often higher for regulated or pharmaceutical orders. Labels outline standard details: batch numbers, molecular weight (115.14 g/mol), CAS number (670-87-1), and safety icons covering irritation and volatility warnings. Each shipment carries documentation on analytical methods—usually GC or NMR profiles—plus certificates of analysis so buyers know exactly what’s in the drum or bottle. Temperature and humidity instructions matter, since marginal contamination can tweak both handling and downstream chemistry. Hazard symbols tell the story of a modest irritant, best handled with gloves and safety glasses, especially in large volumes or high-concentration work.
Synthesizing 3-Ketomorpholine boils down to rearranging morpholine rings and carefully introducing the ketone group. The classical pathway starts with morpholine itself, which reacts under oxidizing conditions—think about agents like potassium permanganate or peracids—to slip in that carbonyl at the 3-position. Sometimes, chemists detour through a two-step process: starting with substituted morpholine, carrying out selective oxidation, and then cleaning up with distillation or column chromatography. It’s easy to mess up; too harsh oxidation brings unwanted by-products or ring cleavage. Yields jump up when the stoichiometry is dialed in and temperature controlled tightly during the addition. Many industrial setups have tuned continuous-flow reactors for the key oxidation, getting better recovery and less waste than old flask methods.
The chemical playground opens wide with 3-Ketomorpholine. Its highly polarized carbonyl brings attention from nucleophiles—amines, alcohols, hydrazines—making it a favorite for forming new C–N or C–O bonds. Reductive amination flips it into substituted morpholines, key to custom pharmaceuticals and crop science tricks. Chemists who want even more complexity use 3-Ketomorpholine as a platform: tack on spicy side chains, extend the ring, or create chiral centers for more specialized drugs. The ring stays resilient under most reaction conditions, tolerating mild acid or base and even some transition metal catalysis. In cross-coupling, especially, the compound has made life easier for med chemists chasing elusive bioactive molecules.
Anyone searching catalogs or academic papers bumps into various aliases for the core molecule. IUPAC goes with 3-Oxomorpholine. Other labels float around: Morpholin-3-one, or even just “ketomorpholine” when context makes things clear. Some distributors stick with the CAS number (670-87-1) or old school short forms—MOR-3-one—for ease of ordering. Trade names haven’t really caught on: this product doesn’t see massive consumer use, so brand terms rarely stick around. Chemists keen on tracing literature need to hunt across all these names for comprehensive background.
No matter how often you use it, 3-Ketomorpholine calls for real attention in safety routines. Skin and lung irritation tops the list; a vapor inhalation won’t kill you but could leave you coughing all afternoon. Spills can burn if left uncleaned, so folks usually work with gloves, goggles, and a good hood fan. Waste goes straight to hazardous disposal. Compatibility with glass and stainless steel means standard labware works fine, no stubborn stains or leaching. Storage away from heat, sunlight, and open air keeps both material and people safer. Labeling remains a must—misidentifying it as morpholine would leave a bad mark on any safety record.
The true strength of 3-Ketomorpholine shows up once projects get moving in diverse fields. In pharma R&D, it’s often a trusted intermediate for producing small-molecule drugs with complex heterocyclic backbones. It feeds into anti-infective, CNS-active, and anti-proliferative scaffolds, offering synthetic flexibility where simple morpholine falls short. Industrially, it appears as a linker or modulator in specialty chemicals, lubricants, and polymer additives, where exact molecular design tweaks performance. Analytical labs value it as an internal standard or reactant for selectivity in method development. Agrochemical makers look at the molecule’s adaptability for prepping crop-protection leads that evade old-resistance trends. Every user pulls different strengths from the same base molecule—a solid sign of a versatile foundation chemical.
Academic and industrial setups both pour resources into 3-Ketomorpholine projects. In my own experience, the compound turned up at the heart of library synthesis, helping postdocs and graduate students test diverse transformations across dozens of functional groups. R&D chemists leverage its ring rigidity and polar backbone to design new drug candidates aiming for potent, selective action in the body. Teams modify the ketone to hunt for new antibiotic leads or tweak solubility for central nervous system penetration. Even material science groups dabble—studying the compound’s ability to serve as a template or nucleator in novel polymer blends. Labs keep pressure on suppliers to refine purity, boost batch-to-batch consistency, and develop derivatives with smarter activity profiles.
Researchers don’t skip toxicity screens for something as reactive as 3-Ketomorpholine. Acute studies in rodents suggest a moderate level of systemic toxicity, so nobody expects this chemical to end up in over-the-counter remedies anytime soon. Irritation risk drives most operational checks, with skin and lung sensitization the main flags. Chronic exposure data runs thin, encouraging teams to run tight workplace safety protocols and stick to gloves-goggles-labcoat routines without exception. Occasional environmental fate work points to partial biodegradation in water and soil, showing less risk than halogen-heavy relatives but still enough persistence to warrant proper chemical disposal.
Looking ahead, 3-Ketomorpholine’s chemistry stays front and center for those needing robust, modifiable N-heterocycles. Drug developers could tap its unique ring framework to sidestep existing resistance in antibiotics or as a base for next-generation CNS therapies. Green chemistry teams keep chasing cleaner, less energy-hungry routes—continuous-flow microreactors, bio-catalysis, even electrochemical methods—to both make and further modify this molecule. Researchers will likely report more specialized derivatives, tailored for both medical and material innovation, and the trend toward tighter regulatory standards should boost demand for higher-purity, traceable production. Open data on long-term safety and environmental slipstream will keep the field honest, pushing new best practices and possibly opening doors to uses nobody predicted when morpholines first caught attention decades ago.
The mention of 3-Ketomorpholine might draw blank stares outside chemistry circles, but its impact stretches farther than most realize. Years ago, I first encountered this compound in an industrial setting focused on specialty chemicals. Workdays often began with meetings about optimizing reactions, and 3-Ketomorpholine stood out because of its versatility. It’s not one of the buzzword chemicals you’ll find in pop science articles, yet it finds real use in places where precision in manufacturing makes all the difference.
The main interest in 3-Ketomorpholine springs from its role as an intermediate. This means that while you won’t see it on the shelves of drugstores or hardware shops, it’s used as a middle step in making much more familiar products. Think pharmaceuticals and agrochemicals. This single molecule can help build more complex substances that show up in everyday medicine cabinets or protect crops in the field. Its unique structure—with both a nitrogen and an oxygen atom in a ring—lets it slip into many different reactions. As someone who’s worked in chemical production, I'm always drawn to compounds with this kind of flexibility because they help cut down on the number of different chemicals needed at a plant, making the whole production pipeline a little less tangled.
3-Ketomorpholine also pops up in the world of research. People who design new drugs constantly look for building blocks that can give a molecule new abilities. Adding a piece like this can unlock improved properties—maybe a longer shelf life for a new medication, or better absorption in the body.
It’s easy to forget all the steps behind things like antibiotics or tried-and-true painkillers—each one involves invisible actors making bits of the final molecule. Watching a team develop a new drug or even a herbicide, there’s always excitement when a lesser-known intermediate makes the process smoother or cuts costs. Industrial chemists lean heavily on compounds like 3-Ketomorpholine, especially when they’re trying to scale up from a lab concept to real-world production. I remember a project where we saved weeks because this intermediate reacted so cleanly, avoiding sticky messes that usually plug up the equipment.
Health and safety matter too. Some chemical intermediates demand hazardous storage or produce toxic byproducts. 3-Ketomorpholine tends to behave better, though anyone in the industry knows to treat all reagents with respect. Factories that rely on cleaner processes and safer chemicals have an easier time meeting environmental rules and worker safety guidelines. On the flipside, I’ve seen what happens when production lines run on old, dangerous intermediates: more spills, tougher clean-ups, slower regulatory approvals. So using something like 3-Ketomorpholine can mean fewer headaches and smoother inspections.
There’s room for improvement. While supply chains for specialty chemicals are more stable than a decade ago, global events or trade disputes can slow delivery. Sometimes, finding a domestic supplier who produces high-quality 3-Ketomorpholine feels like gold mining. Investing in local production would help, especially as more companies try to shorten supply lines after pandemic disruptions. Research labs at universities also play a big part—newer, greener methods to produce 3-Ketomorpholine could cut waste and energy use, both big targets in the chemical industry these days.
Chemicals like 3-Ketomorpholine remain mostly hidden from the public eye, but inside the world of making medicines and advanced materials, every smart choice—every step saved, every risk lowered—affects what ends up in our hands and on our tables. My years in the field taught me to pay attention to these behind-the-scenes players, since industry always leans on good chemistry to get things done right.
Anyone with a bit of chemistry in their background knows morpholine for its odd scent and ring structure. Once you tweak that ring and slap a ketone group onto it, you get 3-Ketomorpholine, a compound that tends to raise eyebrows in chemical circles. The chemical formula is C4H7NO2, which means you’re looking at four carbons, seven hydrogens, one nitrogen, and two oxygens all packed into a relatively simple architecture.
Structurally, 3-Ketomorpholine carries the skeleton of morpholine—a six-membered ring featuring four carbons, an oxygen, and a nitrogen. At the third carbon, it swaps out one hydrogen for a double-bonded oxygen, the hallmark of a ketone. Drop the general formula into a drawing: a hexagonal ring, nitrogen and oxygen sitting across from each other, then tack on that double-bonded oxygen at the third carbon.
Getting down to brass tacks, the shift from morpholine to its 3-keto version changes plenty. In my own years in an organic chemistry lab, I saw how one oxygen atom could send compounds on completely different paths. Adding a ketone at that particular spot doesn’t just check a box—it reshapes how the molecule interacts with others, especially with acids and bases. The molecular tweak changes boiling points, solubility in water, and even the way the ring flexes or reacts with nucleophiles. Those shifts can mean new doors open for making pharmaceuticals, custom catalysts, or specialty coatings.
In synthesis, 3-Ketomorpholine provides a nifty building block. Chemists have a soft spot for compounds like this because the ring strain and unique electron layout can lead to reactions you just can’t pull off with regular morpholine. For example, the presence of the electron-hungry ketone changes how the entire ring behaves under various reaction conditions, making it handy for researchers looking to push the envelope on new chemistry.
Like a lot of specific chemicals, 3-Ketomorpholine doesn’t get to play the lead role in big production runs or industrial synthesis—at least, not yet. Its low profile means it usually pops up as an intermediate on the way to something larger or more complex. Reliable data on toxicity is sparse, and safe handling rules must be drawn from similar compounds. In smaller labs or startups, the cost and difficulty of sourcing rare reagents and the risk of unknown hazards keep 3-Ketomorpholine from wider use.
Folks interested in scaling up work with 3-Ketomorpholine face familiar hurdles: inconsistent supply, tricky purification steps, and gaps in green chemistry methods. My own attempts to make ketone-functionalized heterocycles in graduate school came up against nightmares in purification and waste disposal. Equipment setup often didn’t match the unique reactivity profile, and cleanup was no small concern.
More research into safe handling and reliable synthesis methods could break down some barriers. An updated hazard profile and more robust routes for making 3-Ketomorpholine, possibly starting from renewable feedstocks or using milder conditions, would help. Sharing successful synthesis methods and pushing for better analytical standards could benefit small labs without big budgets. Investment in this area often pays off downstream with new drugs, plastics, and even fine chemicals that play key roles behind the scenes of daily life.
Some chemicals in labs and warehouses draw extra caution for good reasons. 3-Ketomorpholine is one of those. Anyone who’s handled or stored chemicals for more than a few months learns fast that shortcuts lead to headaches—or worse. Vapors, spills, silent reactions behind closed storage doors, each mistakes can turn routine into disaster. My early years in a chemical plant saw enough emergency drills, triggered by forgotten lids or wrong shelf placements, to make careful storage a reflex.
For 3-Ketomorpholine, dry, cool surroundings do more than keep it fit for use. Too much moisture triggers slow breakdown, and what starts small grows into product loss or creates a hazardous soup. Leave it in a warm or sunlit room and you might draw off dangerous decomposition gases. Direct sunlight speeds up these nasty reactions, making the chemical unstable and your workplace dangerous. Every time I saw a bottle of tricky solvent sitting by a window, I remembered the warnings from old-timer lab techs—move it out of the sun or pay for it later.
A locked cabinet makes sense, not just for theft, but to keep out the casual hand. Children, visitors, and even well-meaning coworkers grab from open benches if nobody’s looking. With 3-Ketomorpholine, mistakes can hurt skin and lungs in a matter of minutes.
Original packaging isn’t just corporate habit—it’s a shield. Labels spell out handling steps. Unlabeled flasks in shared spaces still give me pause. Polyethylene or glass containers, tightly sealed, block the slow seep of vapors into storage air. Nothing sharp or brittle. Metal sometimes reacts, so glass earns its keep on this one.
Using the right cap beats improvising. A bad fit turns into a leak that’s easy to miss. Once I spotted a faint, odd smell in a closet—turned out, a barely-misaligned lid started corroding the shelf slow and steady.
Not every workplace remembers their fire plan. 3-Ketomorpholine has a flash point to worry about. It’s not as forgiving as some think. Keep it away from sparks, heaters, and lights with old wiring. I’ve seen small bench fires from less hazardous solvents; clean storage nips trouble in the bud. In industrial labs, separating incompatible chemicals—storing acids, bases, oxidizers apart—helps everyone sleep better at night.
A safety data sheet lives in every good lab. If a bottle tips, if a question pops up, that page spells out steps clear as day. Responders, cleanup teams, whoever’s nearby, can act on real facts instead of guessing or searching online in a panic.
Training matters more than rules taped to a wall. New staff pick up habits by watching others. Walk-throughs, real discussions on mistakes, and calling out what worked, gets the message through. Storage isn’t just books and bins—it’s practicing respect for every bottle’s label and the risks they carry. In my years with chemical warehouses and university stockrooms, those who took the time to store things right never found themselves on the wrong end of a spill.
3-Ketomorpholine earns careful storage—not out of fear, but out of respect for everyone who shares the space.People toss around chemical names so often that they start to lose their edge. Still, 3-Ketomorpholine deserves a healthy dose of respect. This compound isn’t something to just tip into a beaker and forget. It has a reputation for being a stubborn irritant, and my memories of fume hood mishaps have hammered this home. Eyes and skin feel the bite quickly when careless hands drop the gloves or someone forgets to secure their goggles.
Lab notes from years back remind me: this molecule wants out. It evaporates and sneaks into the air if the flask sits open. Lean over a reaction for too long and the vapors make their presence known — sneezing, burning, and that sharp chemical sting at the back of the throat.
Personal protective equipment works best when used without shortcuts. Gloves need to fit without holes, and lab coats keep splashes away from skin and street clothes. Protective goggles rest on my face every time the bottle comes out. One memory sticks with me: a friend tried to pipette a tiny amount for a test, skipped the goggles thinking it was a “quick job,” and spent the afternoon at occupational health rinsing his eyes. 3-Ketomorpholine doesn’t forgive sloppiness.
Ventilation matters. Fume hoods aren’t a suggestion. I’ve watched people try to wriggle out of using the hood to save a few minutes. They regret it after a blast of odor and sore lungs. Keeping the vessel sealed, using the sash, and waiting for exhaust fans to clear the air form habits that save everyone’s nerves.
Locking up chemicals after use turns into second nature. 3-Ketomorpholine likes a cool, dry spot, away from light and anything reactive. Shoved into the wrong cabinet, it becomes next week’s crisis. Mixing with acids or oxidizers spells trouble. Bottles get clear hazard labels and dates, always. Memories of mystery bottles from my undergrad days come back — nobody could figure out what was inside, and disposal cost the lab both time and money. The lesson stuck: label every bottle right after use to avoid someone else’s headache.
Spills demand fast and careful cleanup. Granular absorbents tackle small leaks. Larger messes need pads and extra gear. No one wants chemicals on their skin or soaking into shoes. Getting rid of waste in designated bins keeps the workspace cleaner and cuts down accidents. Quick fixes, like using water to rinse spills, might just spread the problem or send fumes into the air. Chemical-resistant gloves and a clear plan handle these issues much better.
I’ve dealt with new lab members who treat safety protocols as overkill, usually until they see an incident unfold. Stories spread, and sometimes the scare lingers longer than the injury. Training goes far — walking folks through each precaution, showing them scars of past mistakes, and teaching them how to read the safety data sheets with real focus. Respect for chemicals builds over shared cups of coffee, whispered warnings, and the daily routines that keep everyone healthy.
Handle 3-Ketomorpholine with full awareness. Wear the right equipment. Keep things labeled. Use the fume hood even for fast tests. Clean up with purpose. Look out for the next person as much as for yourself. Experience proves that these simple steps don’t slow down science — they protect it.
Scientists and chemists sometimes take for granted how easy it is these days to order all kinds of chemicals online. You need something for research, you pull up Sigma-Aldrich or TCI, and likely it’s in stock. Not every molecule plays along, though. Take 3-Ketomorpholine—a simple name but it doesn’t pop up even after searching all the usual catalogs. I remember running into a similar situation with a rare lactam two years ago, and it felt like looking for a white crayon in a box of only black ones.
Major sellers like Merck, Fisher, and Alfa Aesar don’t keep 3-Ketomorpholine on hand. Requests turn up nothing—no listing, no backorder, not even a “contact us.” In lab circles, that usually hints at something: the compound either has limited interest, presents tricky handling, or faces regulatory hurdles. You won’t even spot research chemical suppliers listing it outside obscure forums, and those links vanish after a while. From experience, anything this hard to find probably hasn’t caught on commercially—either the chemistry is too cumbersome, or no industry application pulls its weight.
Any compound with a niche structure or unknown safety profile might be risky for suppliers. I’ve had accounts flagged over more common precursors just because law and policy folks watch out for new psychoactive substances or synthetic intermediates. They clamp down, sometimes too harshly, just to keep ahead of possible misuse. Some even filter search traffic—try looking up seed oils one week, and the next, you’re stuck answering a compliance officer’s phone call. 3-Ketomorpholine has the sort of structure that triggers extra questions. No surprise, then, that vendors steer clear.
Facing a dead end on the supply front, some labs gear up to synthesize their own. This route chews up time and money. You’ll need access to morpholine, oxidizing reagents, and probably a skilled hand at purification. I’ve worked with morpholine derivatives in my old lab, and just getting clean product often needed half a week—plus the risk of exposure to nasty by-products. Add tight waste disposal rules and safety reviews, and suddenly making grams of this stuff looks like more trouble than it’s worth for most outfits. Plus, unless you’re looking to publish or prove a process, home-brewing isn’t an easy sell to project managers.
Industrial buyers chase reliability. They need safety data sheets, bulk pricing, and hazard clearances. Without those, contracts stall and projects sit idle. Smaller labs can sometimes scrape together a supply through academic connections, or look for a custom synth job, but that brings new headaches: lead times drag on, minimum order quantities balloon, quotes land in the five digits. I’ve been quoted $10,000 for a single rare heterocycle once—not counting shipping or customs.
Laboratory chemistry would benefit from more transparency around rare intermediates. Suppliers might flag what they're legally blocked from selling, and maybe build a portal for researchers to signal demand. Collective requests sometimes nudge catalog managers to stock a hard-to-find compound, or even set up a group buy. Scientists can pressure publishers to require sourcing statements in their methods, which helps everyone know what’s possible without detours and guesswork.
Stumbling over a molecule like 3-Ketomorpholine pushes teams to collaborate, improvise, or seek alternatives. Sometimes, it even sparks new research angles—not a dead end, just a sharp detour on the journey.
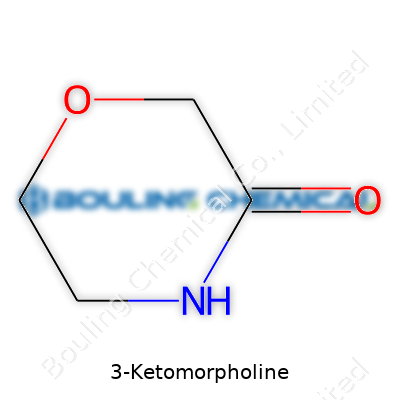
| Names | |
| Preferred IUPAC name | morpholin-3-one |
| Pronunciation | /ˌθriːˌkiːtəʊˈmɔː.fəˌliːn/ |
| Identifiers | |
| CAS Number | 670-47-1 |
| Beilstein Reference | 1180714 |
| ChEBI | CHEBI:59041 |
| ChEMBL | CHEMBL47820 |
| ChemSpider | 10270539 |
| DrugBank | DB08294 |
| ECHA InfoCard | 36b7e880-78e4-45ee-81b0-18e5ced993c8 |
| EC Number | 3.5.2.13 |
| Gmelin Reference | 115860 |
| KEGG | C21155 |
| MeSH | D034607 |
| PubChem CID | 13941 |
| RTECS number | QU4375000 |
| UNII | 0JEC40415X |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C4H7NO2 |
| Molar mass | 129.16 g/mol |
| Appearance | White to light yellow liquid |
| Odor | Amine-like |
| Density | 1.14 g/cm3 |
| Solubility in water | soluble |
| log P | 0.04 |
| Vapor pressure | 1.34E-02 mmHg at 25°C |
| Acidity (pKa) | 6.95 |
| Basicity (pKb) | 7.94 |
| Refractive index (nD) | 1.495 |
| Dipole moment | 7.07 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 240.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -482.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | –3682.6 kJ/mol |
| Hazards | |
| Main hazards | Causes skin irritation, causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06, GHS08 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-3-0 |
| Flash point | 110 °C |
| Autoignition temperature | 270 °C |
| Explosive limits | Explosive limits: 3.4–11.4% (in air) |
| Lethal dose or concentration | LD50 (rat, oral) 980 mg/kg |
| LD50 (median dose) | 580 mg/kg (rat, oral) |
| REL (Recommended) | 20~25°C |
| IDLH (Immediate danger) | IDLH: Not Listed |
| Related compounds | |
| Related compounds |
Morpholine N-Methylmorpholine 2-Ketomorpholine 3-Hydroxymorpholine 4-Ketomorpholine |