People have searched for new building blocks in organic synthesis for many years. 3-Iodothiophene came out of that search, carving a path through academic and industrial chemistry. In the late 20th century, the boom of cross-coupling technology—think Suzuki and Heck reactions—gave iodo-substituted heterocycles a new spotlight. Researchers focused efforts on iodinated thiophenes since they offered reactivity without too much fussiness in the lab. Today almost every major catalogue stocks 3-iodothiophene, which says a lot about its journey from obscure compound to crucial chemical.
3-Iodothiophene looks pretty unremarkable: clear to slightly yellow liquid, sharp, sometimes a little pungent. Lab workers might remember the first whiff and the stubborn stain on gloves. In synthesis, it serves as a platform—an entryway for making more complex molecules through well-developed transformations. Its ready reactivity at the iodine position and five-membered ring gives chemists a shortcut, often saving whole steps in synthesis compared to other derivatives. You find it bottled as a standard reagent, usually at purities around 97% or higher, which supports applications in research and manufacturing.
This compound weighs in at 225.06 g/mol. Its boiling point sits near 73–75°C at reduced pressures, useful for anyone distilling or handling under vacuum. In the fridge or a cold room, it stays stable and resists decomposition. It dissolves well in organic solvents: ether, tetrahydrofuran, dichloromethane, and even acetone. The electron-withdrawing iodine alters the ring’s chemistry, slowing direct electrophilic reactions but making it perfect for metal-catalyzed couplings. You get a soft, non-crystalline liquid, not much odor but enough to let you know you’ve spilled some.
Manufacturers provide plenty of data: purity levels, NMR spectra, GC traces, and safety warnings. Bottles come labeled with batch numbers and storage recommendations, sometimes nitrogen or argon-sealed if the supplier expects a long journey. Chemists check CAS number 5722-19-2 to avoid mix-ups with other iodothiophenes. In-house procedures dictate that stock bottles stay cool and dry, with logs to limit cross-contamination. The melting point hovers below room temp so no fiddling with solid-phase scoops.
People make 3-iodothiophene by direct iodination of thiophene, which takes careful control—you want the iodine to hit the right spot. Older protocols stretched out, requiring protection of functional groups and specialized solvents. Over time, improvements shortened these syntheses to one or two steps, with N-iodosuccinimide or molecular iodine in acidic media. Sometimes organolithium routes or directed ortho-metalation offer cleaner routes for those with more resources. Large-scale producers often select the safest, easiest method to minimize byproducts and keep plant workers safe.
Anyone with experience in organic synthesis knows the power of halogenated aromatics. 3-Iodothiophene slots right into palladium- or copper-catalyzed cross-coupling, turning into diverse biaryl or alkenyl-thiophenes. You get direct access to Suzuki, Sonogashira, or Stille products, making it a favorite in pharmaceutical discovery. The site-selective substitution allows for heavier groups, such as aryl, vinyl, or alkynyl, while preserving the sulfur ring. Some use it for carbonylation or amination, seeking new lead compounds. In the hands of a creative chemist, it unlocks unique heterocyclic derivatives—hard to build any other way.
Science catalogs carry it under several names: Thiophene, 3-iodo-, 3-Iodo-thiophen, and more trivial monikers like m-Iodothiophene. Searching by CAS number clears up any ambiguity. Brand names may add catalog or batch codes; Sigma-Aldrich, Alfa Aesar, and TCI list it under standardized titles, always pointing back to the same core structure.
Work with 3-iodothiophene brings familiar hazards. Liquids seep through gloves if handled carelessly, and vapor inhalation isn’t wise—though not acutely toxic, repeated exposure to organoiodine compounds can’t be brushed off. Standard precautions include lab coats, nitrile gloves, and fume hoods. Fire risk stays moderate, mainly from solvents used alongside. In real labs, spill kits and eye wash stations stand ready. Material safety data sheets (MSDS) warn of acute effects like skin or eye irritation and suggest storing away from oxidants or direct sunlight. Chemical waste streams collect unreacted starting materials and halogen-containing byproducts for proper disposal.
3-Iodothiophene shows up everywhere from academic research to specialty material manufacture. Medicinal chemists reach for it when they need quick modifications on thiophene rings in drug scaffolds. Solar cell researchers test it as a precursor in building block units for organic semiconductors and conductive polymers. Material scientists have built new OLED prototypes and organic field effect transistors by modifying thiophene moieties starting at the iodo stage. Agrochemists use it as a stepping-stone for fungicide and pesticide synthesis, especially when activity depends on substitution pattern around the ring.
Interest in 3-iodothiophene stays high in method development. Synthetic organic chemists study new ways to cut down on waste and eliminate expensive catalysts. There’s a current wave of work on greener iodination protocols and non-toxic transfer reagents. Some labs experiment with flow chemistry to scale up cross-coupling efficiency. Medicinal groups screen 3-iodothiophene scaffolds in anti-cancer or anti-inflammatory drug leads, following activity clues from computational models. Material science research pushes the boundaries in polymer backbone engineering, seeing whether new derivatives outperform older compounds.
For toxicity, studies go deeper than basic irritation profiles. Chronic exposure studies explore the risk from halogenated aromatics, with some evidence suggesting mild but not severe toxicity from short-term exposure. Animal tests chart acute LD50 values that show moderate risk levels, not nearly as alarming as polychlorinated organics but still requiring respect in the lab. People occasionally report allergic reactions or sensitization after repeated contact—especially in those working with heterocyclic halides over long periods. Waste policies reflect this risk, sending anything containing the iodo group to specialized incineration rather than down the drain. Biosafety teams help train newer workers, ensuring good habits are carried forward in research institutions and chemical manufacturing plants.
The outlook for 3-iodothiophene hinges on two main ideas: enabling new synthetic chemistry and advancing electronic materials. As pharmaceutical companies chase diverse new scaffolds, efficient ways to modify thiophene rings make a big difference. Environmental regulation and the drive toward green chemistry force companies and academic labs to seek out less hazardous preparation and handling methods. The trend points toward automated synthesis platforms, robotics that can manage multiple cross-couplings from a central stock of building blocks like this. In green energy and electronics, demand keeps rising as researchers try to squeeze every ounce of performance out of next-generation materials. With the cycle of discovery rolling on, 3-iodothiophene keeps earning its place on the shelf, bridging practical chemistry and cutting-edge technology.
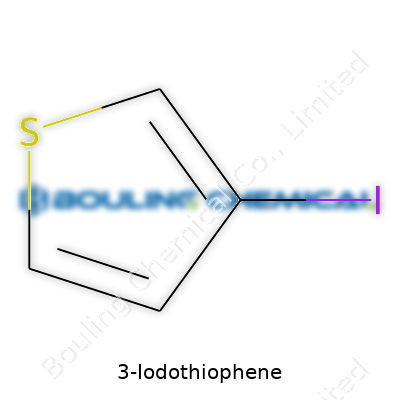
3-Iodothiophene doesn’t pop up during your daily routines or coffee breaks, but its molecular formula—C4H3IS—tells a detailed story. Each letter and number speaks volumes to chemists drawing up reaction plans or materials scientists pushing to create the next flexible screen or solar cell. This formula shows just how a subtle switch—a hydrogen traded out for an iodine at the third carbon—reshapes what the basic thiophene ring can do. Just like in cooking, changing a spice can totally remake a familiar dish.
Add an iodine to the thiophene core and things change fast. As I learned while spending nights in the organic chemistry lab, that single iodine does more than add weight. Iodine grabs attention on this molecule—it's big, polarizable, and attracts the eye of anyone tasked with building new compounds. Compared to bare-bones thiophene (which swings by as C4H4S), this iodo version isn’t shy about joining new reactions. That’s key for making smart drugs, new polymers, and materials that actually do more than just sit on a shelf.
Lab folks often talk about molecules like old friends. Some are wildcards, others are reliable workhorses. Iodothiophenes pop up often at the crossroads of invention. Want to build up a new bioactive compound for cancer trials? The recipe often starts with a halogenated ring because it snaps into place with reagents that seem too picky. These rings make it possible to build libraries of test compounds quickly. And if you work in electronics—think screens that bend or microchips that sense tiny chemical changes—those sulfur and iodine atoms bring in properties you won’t get from plain rings.
I remember hearing colleagues struggle when supply chains choked off access to key chemicals. Iodine isn’t always easy to find or cheap to source, especially in regions heavily tied to global trade hiccups. The environmental baggage of extracting iodine adds weight, with mining and processing creating headaches for those who care about their ecological footprint. As chemists and engineers look at the map of the periodic table, alternatives always matter. Green chemistry—using more benign reagents or recycling precious halogens—can take some sting out of these issues.
To many, molecular formulas look like a bedtime story for scientists. But C4H3IS, the formula for 3-iodothiophene, means efficiency for the researcher racing to synthesize a molecule for an unpublished patent or a promising medication. The way iodine slots onto that ring streamlines further transformations, and for those of us who have been in synthesis, saving even a step or two can turn months into weeks. Still, every easy shortcut with heavy atoms carries a price—both financial and for the planet.
Chemists face pressure to keep innovating, but no one ignores supply bottlenecks or environmental problems for long. Smarter approaches might mean revisiting less conventional halogens or shifting towards more sustainable feedstocks. Universities and companies backing green chemistry efforts could push for incentives that make clever substitutes available on the same scale as traditional halogens. Makers and thinkers on the ground end up driving the change.
My time working with chemical research teams taught me about the hunt for chemicals that spark real progress. 3-Iodothiophene is one of those candidates. This sulfur and iodine-containing compound, with its quirky ring of atoms, shows up again and again when labs go looking for new paths in organic chemistry. It’s not just a curiosity — it proves its worth as a starting block for building larger, more complex molecules.
Ask almost any medicinal chemist about halogenated thiophenes, and you’ll see their eyes light up. The pharmaceutical industry often depends on these types of building blocks. 3-Iodothiophene steps in for cross-coupling reactions, especially Suzuki and Sonogashira couplings, which let researchers bolt interesting pieces onto it. This isn’t just textbook stuff. Real-world painkillers, anti-viral agents, and anti-inflammatory drugs have roots in thiophene chemistry. Pharmaceutical patents from Pfizer, Roche, and others talk about their reliance on iodothiophenes when creating drug scaffolds.
From experience, speeding up the discovery of a promising compound doesn’t just help companies — it can mean life or death for patients waiting on the next big treatment. Synthesis that starts with 3-Iodothiophene opens routes that would stay closed with more rigid chemicals.
During a stint in an electronics start-up, I saw just how much organic electronics owe to these molecules. 3-Iodothiophene ends up being more than just a chemistry trick: producers working on OLED screens and organic solar panels frequently build on thiophene backbones. Adding an iodine atom changes the way these rings stack and interact, letting designers tweak how films absorb and emit light.
It’s not all about theory. Real displays glow to life because someone figured out how to control the arrangement of molecules. This chemical lets researchers connect thiophene units where they want, leading to new conductive polymers. These polymer chains wind up as the films inside next-gen TVs, flexible displays, or even biosensors in hospitals.
Anyone who’s struggled to make a custom molecule knows the value of a shortcut. 3-Iodothiophene turns into countless derivatives, including ones with carboxyl, aldehyde, or even other halogen groups. Custom ligands for catalysts, rare intermediates for dyes, or tools for agricultural chemical discovery — all start with small tweaks to the core molecule.
Chemists keep reaching for iodothiophenes, not because they fill a shelf, but because they save time and help sidestep dead-end steps that waste effort and money.
As excitement over greener chemistry grows, some ask tough questions about the raw materials we all use. There’s been movement toward making iodothiophenes with less impact, cutting out harsh reagents or energy-heavy setups. Flow chemistry and photo-driven reactions are popping up in journals, promising more sustainable options.
Every field could use easier access to the right starting blocks. Grants and technical conferences I’ve attended echo the same message: tools for cleaner production can close the loop, sparking new discoveries in both labs and on factory floors.
Whether it’s the next treatment in the pharmacy or a slicker phone display, breakthroughs depend on letting researchers build complex things from simpler parts. 3-Iodothiophene plays this part well, and chances are, its headline role in science and industry isn’t going away soon.
People who work with organic chemicals value purity more than flashy advertising or eye-catching packaging. In labs and production lines, clean reactants mean fewer unpredictable results, better yields, and safer working conditions. I look at 3-Iodothiophene—an aromatic compound with iodine tucked onto a thiophene ring—and the first thing I check is the label for purity, not the logo.
Shops and global suppliers, from Sigma-Aldrich to TCI and Alfa Aesar, commonly sell 3-Iodothiophene at 97% or 98% purity. For reference, I have a habit of flipping through catalogs and online listings out of curiosity whenever I’m waiting for a reaction to run. Most reference-grade batches used for research hover between 97 and 99 percent purity. On occasion, pharmaceutical-grade or ultra-pure versions turn up, but these cost twice as much and rarely show up in teaching labs or routine organic synthesis.
This isn’t just an arbitrary choice. Many synthetic chemists weigh the benefits of higher purity against bigger expenses and stricter handling requirements. For exploratory or early-stage reactions, 97% is often enough to screen ideas and pathways. They rarely throw money at 99.99% unless a trace byproduct threatens the whole project.
I’ve seen what happens when someone skips over purity checks. Lower-quality batches—anything below 96%—bring headaches during column chromatography and NMR. Side products and leftover reagents muddy the final product and muddy spectra. I remember one graduate student who spent days troubleshooting an unexplained bump in her product yield. Turns out, the reagents she’d ordered came with unexpected contaminants, and the solution involved a lot of calls and some embarrassing explanations during lab meetings.
High-purity 3-Iodothiophene is especially important for industries making advanced pharmaceuticals, OLEDs, and specialty polymers. These fields don’t tolerate mystery peaks during quality control. If a single batch of contaminated reagent gets into the system, someone has to clean up not just the immediate mess, but also the unreliable data and wasted weeks of effort.
Getting pure 3-Iodothiophene shouldn’t feel like a scavenger hunt. Suppliers post technical sheets and offer certificates of analysis for every batch. Researchers who actually check these details avoid surprises later. If purity looks questionable or the supply chain feels shaky, contacting the vendor usually helps. Some even offer fresh testing for a fee—something many labs ignore, but the option’s always better than guessing.
One idea I’ve picked up from seasoned chemists is pooling orders or sharing bulk purchases with neighboring labs. This cuts costs and virtually guarantees fresher stock. Another promising step is asking vendors for smaller samples if larger batches feel risky. This is handy for trial runs or people aiming to minimize waste and reduce storage headaches.
In the end, selecting the right purity feels less about hitting a specific number and more about matching the reagent to the goal. If an experiment depends on fine margins, that extra cost for higher-grade 3-Iodothiophene can save weeks of troubleshooting. For routine experiments, standard 97% or 98% grades usually hold up just fine.
Anyone who works in a chemical lab knows the unruly side of certain chemicals. 3-Iodothiophene earns respect. It’s a volatile, strong-smelling compound with a flair for reacting with air and light. I remember my first encounter in a cramped organic synthesis lab, whiteboard full of reaction schemes, and the bottle of 3-Iodothiophene tucked in a corner. My supervisor glanced at the bottle and casually warned—keep it away from sunlight and moisture or risk spoiling an expensive batch.
3-Iodothiophene doesn’t foul up by accident. Expose the liquid to air for too long, and it starts degrading, releasing subtle but unmistakable hints of iodine. That signals the start of trouble. Moisture slips in, hydrolysis follows, and pretty soon you have a bottle that’s useless for even the boldest synthetic chemist.
I’ve seen research budgets stretch thin because a poorly stored bottle lost its punch. Setting up a reaction, you expect sharp NMR peaks, but the spectrum comes back messy—turns out, contaminated starting material is often the culprit.
Don’t leave 3-Iodothiophene on an open shelf or near a window. This isn’t table salt, it demands a cool, dry, dark home. Storage away from strong light cuts down the risk of photodegradation. I keep it in a refrigerator set just for chemicals (usually between 2°C and 8°C), not mixed with samples for organic extractions or food. This chemical hates fluctuations—leave the fridge door open too long and condensation creeps in.
Pretty glass bottles look fine in textbooks, but for real-world use, switch to amber glass or dark polyethylene containers. These block light, especially ultraviolet, keeping the 3-Iodothiophene protected. In our lab, every bottle gets a clearly marked sticker: date received, date opened, and initials. No guesswork about who last opened the bottle, especially after a long weekend.
Tight lids are the frontline defense. A loose cap undermines even the best fridge. I always make sure the rim is completely dry before sealing, since moisture on the thread can sneak inside. Silica gel packets inside the storage cabinet add another layer of protection. While working on a time-sensitive project, I once skipped this step—after a month, the brown liquid turned cloudy and the smell sharpened. Lesson learned.
One thing overlooked is proper labeling. No matter how experienced you are, there will be days when every bottle seems to blur together. Clear, resistant labels cut those mistakes in half. I use chemical-resistant pens, nothing wipes off, and double-check dates before grabbing a working sample for a reaction.
Individual researchers and small labs can’t always rely on automated environmental chambers. So, keeping a dedicated, manageable-size fridge becomes a lifesaver. Split up larger quantities into smaller vials. This means you’re only exposing a small portion of the chemical at a time, reducing total wastage.
I often advise new students—don’t treat these precautions as burdens. Maintaining a robust routine for storing 3-Iodothiophene saves time, money, and frustration. Stopped reactions, wasted reagents, and reordering delays add up. Respect this simple bottle, and it’ll serve your research much longer.
3-Iodothiophene shows up in the world of chemistry labs and industrial synthesis, especially when people work on building complex molecules for medicines or electronics. This compound carries an iodine atom attached to the thiophene ring, making it pretty useful as a building block. Folks who have handled organic chemicals before know that even small changes in a molecule can mean a big shift in risk.
If you’ve spent time around reagents and solvents, you quickly learn to pay attention to their safety sheets. For 3-iodothiophene, those sheets don’t waste words. The compound’s not flammable in the classic sense like ether or acetone. Still, it can produce nasty vapors if heated. The smell signals danger pretty fast — anyone who’s ever inhaled something pungent in a lab remembers the lesson.
Touching this stuff directly on the skin could cause irritation and redness. Vapors, if concentrated, might irritate a person’s airways or eyes. Breathing chemical fumes isn’t a joke — people who work with such liquids should never shrug off the need for good airflow.
3-Iodothiophene’s risks sit somewhere between routine solvents and the more dangerous brominated or iodinated compounds. Mishandling can still lead to chemical burns or inhalation damage. Splash goggles and gloves, especially nitrile, stay on for good reason. Splashing a little on the bench won’t start a fire, but ignoring that spill could mean skin exposure or a surprise when you go to wipe it up and get a faceful of vapor.
Years back in a small research group, I watched a grad student pour a similar compound into a flask — no gloves, just chasing time. Minutes later, his hand turned red, not from pain but a mild allergic response. We spent ages re-teaching safe handling after that. It only takes once.
Global safety agencies tend to label chemicals like 3-iodothiophene under “irritant” and “dangerous for the environment.” Waste gets boxed up and treated with extra care. You can’t just dump solutions down the drain, because iodine-containing compounds create problems at wastewater plants and in groundwater. Even small spills need careful cleanup with proper absorbents.
For those new to working with aromatic iodides, they should never rely on casual habits. Respirators may sound heavy-handed, but splash-prone setups, leaky bottles, or broken glass make a mask a lifesaver. Long sleeves, closed shoes, and alertness in cramped workspaces keep people safe.
Prevention means labeling everything clearly and storing these bottles away from incompatible chemicals — not next to acids or strong bases. Ventilation hoods catch escaping vapors and keep the breathing zone clear. Most labs run annual safety refreshers, but the best education sticks in daily routine: open slowly, use a pipette carefully, double-check cap seals, have spill kits within reach.
For larger productions, automation and sealed processes take human error down several notches. robots won’t risk eye irritation or forget gloves.
Ultimately, 3-iodothiophene doesn’t rust metal or detonate on contact with air, but real risks exist. Long-term exposure hasn’t been studied in detail, but anything that irritates on contact shouldn’t be handled barehanded or open to the air. The culture of safety, not just equipment or rules, lowers risks more than anything.
| Names | |
| Preferred IUPAC name | 3-iodo-1-benzothiophene |
| Other names |
3-Iodothiophene 3-Iodothiofene |
| Pronunciation | /ˌaɪ.oʊ.də.θaɪ.oʊˈfiːn/ |
| Identifiers | |
| CAS Number | [928-68-9] |
| Beilstein Reference | 1203996 |
| ChEBI | CHEBI:51721 |
| ChEMBL | CHEMBL38237 |
| ChemSpider | 33154 |
| DrugBank | DB01938 |
| ECHA InfoCard | ECHA InfoCard: 100.017.852 |
| EC Number | 615-941-5 |
| Gmelin Reference | 731535 |
| KEGG | C07278 |
| MeSH | D016708 |
| PubChem CID | 70347 |
| RTECS number | XN5300000 |
| UNII | W7L6707U6U |
| UN number | 2810 |
| CompTox Dashboard (EPA) | DTXSID4036875 |
| Properties | |
| Chemical formula | C4H3IS |
| Molar mass | 229.01 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | aromatic |
| Density | 2.18 g/mL at 25 °C |
| Solubility in water | Insoluble |
| log P | 1.97 |
| Vapor pressure | 0.00712 mmHg at 25°C |
| Acidity (pKa) | 3.55 |
| Basicity (pKb) | -4.35 |
| Magnetic susceptibility (χ) | -73.0e-6 cm³/mol |
| Refractive index (nD) | 1.663 |
| Viscosity | 2.184 cP (25°C) |
| Dipole moment | 1.63 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 198.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -22.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2448 kJ/mol |
| Pharmacology | |
| ATC code | '' |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H312 + H332: Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 72°C |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10-30°C |
| Related compounds | |
| Related compounds |
Thiophene 2-Iodothiophene 2-Bromothiophene 3-Bromothiophene 2-Chlorothiophene 3-Chlorothiophene |