Chemistry stories rarely start with a dunk in a magic solution and a patented name. The path of (S)-1-tert-Butyloxycarbonyl-3-hydroxypiperidine gives a steady glimpse into how lab benches slowly become launching pads for wider progress. By the late 20th century, as drug designers and peptide chemists began wrangling with protecting groups and searching for chiral building blocks, the piperidine ring, with its close resemblance to natural amino acid backbones, earned a place in many research plans. The (S)-enantiomer first made headlines in peptide synthesis, as folks wanted to tuck away functional groups without triggering side reactions. After several years of trial, error, and cataloging by specialty chemical companies, its protected form, in combination with new purification and chiral separation methods, quickly expanded into global research collections and big pharma stockrooms.
(S)-1-tert-Butyloxycarbonyl-3-hydroxypiperidine usually shows up as a white to off-white crystalline solid, distinct for its chiral purity and role in stepwise assembly of complicated molecules. Its N-Boc protection on the ring nitrogen shields the molecule during tough synthetic protocols, making life easier for chemists racing against decomposition or unintended reactivity. The primary feature: it lets other pieces of a new drug scaffold come together without unwanted side reactions. Multiple catalogues now carry the compound; major suppliers package it with purity ranging from 98%–99% and enantiomeric excess right above 99%, a standard that hints at the jump in demand for regulatory-grade active ingredient starting points.
In practice, (S)-1-tert-butoxycarbonyl-3-hydroxypiperidine has a melting point between 81°C and 85°C. The piperidine ring, protected at the nitrogen by a Boc group, brings some steric bulk and lowers the boiling volatility. Chemists handle it as a stable intermediate under ambient conditions. It dissolves in most organic solvents like dichloromethane or ethyl acetate, which means that both extractions and reactions stay manageable without oddball solubility issues. Its reactivity pinpoints to either the free alcohol at position 3 or at the carbamate linkage, which allows various routes for modification or deprotection depending on downstream plans.
Commercial bottles hit the lab bench labeled with CAS numbers, batch data, and spectral data to ensure identity. Suppliers often provide HPLC chromatograms and optical rotation, establishing chiral purity. Storage calls for a cool, dry location away from strong acids or bases. Each bottle carries a lot number to backtrace shelf history in case of supply chain glitches or regulatory queries. Below these details, product sheets typically mention moisture content limits, residual solvents, heavy metal content, and labelling that makes the line between research-grade and GMP-grade obvious to lure in buyers with different budgets or compliance targets.
From my experience in synthetic labs, the journey starts with inexpensive piperidine, which undergoes enantioselective hydroxylation. Protecting the nitrogen with tert-butyl dicarbonate (Boc2O) follows. The chiral auxiliary or asymmetric catalyst is the key—whether using a Sharpless dihydroxylation or employing biocatalysts for the initial step. After the protection, chromatographic purification becomes a non-negotiable checkpoint, driving up costs but ensuring consistent reproducibility. This stepwise assembly, though time-consuming, wins favor for delivering high optical purity, letting medicinal chemists trust in the upstream integrity of their intermediates.
Once inside the reaction flask, this molecule plays a versatile role. The free hydroxyl on position 3 opens doors for etherification, esterification, or oxidation to form carbonyls. During peptide coupling, the Boc group is removed by standard acids like trifluoroacetic acid, converting the molecule into a functionalized amine ready for more coupling or ring expansion. Functionalization options expand into phosphorylation or even subtle deuteration, letting scientists probe molecular dynamics or fine-tune pharmacokinetic profiles in new drug candidates. Selective activations and clean deprotections keep synthetic planning straightforward, which means cycle times compress and bottlenecks shrink—a feature any process chemist appreciates.
People have a field day with naming in this space. "Boc-(S)-3-Hydroxypiperidine", "N-Boc-(S)-3-piperidinol", and "tert-Butyl (S)-3-hydroxypiperidine-1-carboxylate" all refer to the same compound. CAS number 131108-27-1 shields everyone from confusion in procurement databases. Some niche suppliers tack on proprietary catalog codes but the underlying product rarely shifts in structure.
Handling this piperidine derivative stays pretty straightforward for anyone used to fine organic intermediates. Industry practices recommend gloves, eye shields, and fume hoods to avoid any skin sensitization or respiratory uptake, even though acute toxicity sits low on the scale compared to alkyl halides or isocyanates. Emergency data sheets quote a low risk category, but as with any chemical with protecting groups, decomposed byproducts can become a mess if overheated or stored sloppily with acids. Waste disposal funnels the residue into halogenated solvent streams to avoid dumping Boc derivatives straight into general chemical waste, a lesson many academic labs have learned the hard way following regulatory fines for improper segregation.
Research chemists put this molecule through its paces in the pharmaceutical sector. Its chiral piperidine scaffold fits into the assembly of β-lactam antibiotics, CNS drugs, and enzyme inhibitors. The protected amine functionality slots into combinatorial libraries, streamlining lead optimization campaigns. Its use as a starting material for peptidomimetics lands it on the bench in polymer-supported solid phase synthesis. In contract drug manufacturing, big batches often land in reaction trains for cancer, metabolic, or immune-modulating drug candidates seeking a balance between biological activity and safety profile.
Researchers keep finding new wrinkles to exploit. The main push now revolves around finding greener, more scale-friendly methods than classic organic routes, for both cost and environmental reasons. Electrochemical oxidation and enzyme-based transformations take center stage in process R&D. Teams hunt for milder or more selective catalysts, since large-scale failures bring expensive reprocessing. Regulatory pressure for lower impurity content—especially residual solvents or metals—nudges every process tweak toward cleaner, more robust approaches, opening more doors for academic–industry partnerships and technology transfer.
Toxicology runs in the background for every new intermediate, and this Boc-protected piperidine earns ongoing scrutiny. Short-term studies put it in the low-to-moderate oral toxicity group with little evidence for mutagenicity or teratogenicity. Its byproducts, especially after strong acid deprotection, raise more concern since tert-butyl carbamate can yield isocyanates under harsh conditions—an acute inhalation hazard. Chronic exposure data lag behind, so any process lab sees ongoing monitoring. Waste stream analysis, coupled with LEED-compliant engineering controls, ensures no accidental environmental buildup, especially near water discharge routes.
Big opportunities on the horizon trace back to both better process chemistry and shifting market demand. With more focus on chiral small molecules to address antibiotic resistance, cancer, or neurological diseases, the need for high-purity, easily-modified intermediates rises. Companies exploring continuous flow chemistry or in situ protection/deprotection control turn to staple molecules such as this to trim production times and costs. Underpinning all future directions: a hunger for sustainable, low-waste processes that link synthetic utility to long-term viability in development pipelines.
Mention “(S)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine” to anyone who’s spent time at a lab bench, and you’ll see a faint recognition. This isn’t a household word, unless your household keeps a few Erlenmeyer flasks handy. What we have here is a protected piperidine derivative, a handy tool for chemists piecing together complex molecules, especially in the world of pharmaceuticals.
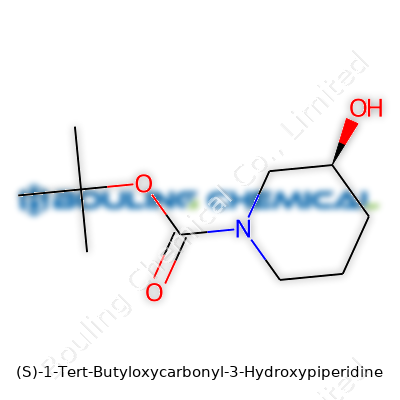
Breaking it down: piperidine forms the core—a six-membered ring, five carbons and one nitrogen. The “3-hydroxy” part means there’s an -OH group sticking out at the third position of this ring. At the nitrogen, we find a bulky tert-butyloxycarbonyl, also known as Boc. This chunk is more than just a mouthful. It keeps the nitrogen out of trouble during certain reactions. In plain language, the chemical wears a cap until we’re ready to use its amine in a later step.
The molecular formula is C10H19NO3. The Boc group contributes a lot to this count. Breaking it down: the tert-butyl part is C4H9, the carbonyl (CO) connects to both the tert-butyl and the nitrogen. You add water-like -OH at position three on the ring. Once you sketch it all out, it forms a pretty fortress around the active parts of the molecule, which matters a whole lot if you want to keep certain reactions quiet and others loud.
It’s not just some chemistry curiosity. This protective group strategy lets scientists build molecules step by step, blocking troublemakers until their turn comes up. A friend who spent long nights working on drug candidates always talked about the frustration when side products crashed his plans. Using the Boc group, he could plan ahead, keeping unnecessary drama to a minimum. The fact is, synthetic chemistry finds success in these small details, not grand inventions.
I remember one organic synthesis class where every side reaction meant hours lost and spirits sunk. Structure solves a lot of grief. The Boc group’s bulk shields the nitrogen, so even a rookie can run reactions at the third position—where the hydroxy group lies—without risking messes on the nitrogen. This focus on structure makes molecules like this super valuable for researchers. It’s always about planning: protect one functional group, manipulate another, then gently remove the shield. It sounds simple. Anyone who’s gone through the deprotection step on the bench knows it’s not snap-your-fingers easy, but without structure control, it’s near impossible.
Labs run on tight schedules and budgets. Wasting time purifying byproducts from a lack of molecular control can sink a whole project. That’s not just theory. I’ve seen people burn through week after week, hoping to squeeze out a little product from a gummy mess. The Boc group, by protecting that amine, lets chemists fine-tune how the molecule behaves. It’s all about choosing the right battles. Shield a site here, open it up there, and suddenly you have a molecule ready for the next synthetic adventure—maybe one leading to a new painkiller, or even a small tweak in an agrochemical.
In the end, the molecular formula C10H19NO3 and that carefully considered structure carry a weight that goes beyond textbook diagrams. Every bit of planning, every strategic shield, gives real people extra hours and more successes in labs everywhere.
You won’t spot (S)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine on a pharmacy shelf, yet this compound gets a lot of respect in the world of chemical manufacturing. Looking at its name, most folks might tune out, thinking it sounds like something meant only for experts, but its uses reach right into the core of how society brings new drugs and treatments to life.
This compound often pops up in the labs where scientists design and test molecules for new medicines. Chemists love using building blocks like (S)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine because it helps control chirality—a concept that’s tricky but, in simple terms, helps decide how a drug interacts with the body. Imagine your left hand versus your right. Both look similar, but only one fits into a glove made for the left hand. Just the same, a drug’s left- or right-handed shape can decide if it works as intended or not.
This molecule offers what’s called a “Boc” group (tert-butyloxycarbonyl). Think of the Boc group like a mask. It temporarily blocks a part of the molecule from reacting until chemists get the rest of their construction finished. Once they’re ready, they can remove this mask with a simple chemical wash, freeing up the molecule for final tweaks. For researchers, this flexibility is gold.
I remember watching a colleague working late in a university lab. He smiled about the relief Boc-protected piperidines bring, describing them as “saving weeks of headaches” during the careful step-by-step assembly of trial medications. The winning point: piperidine structures like this one show up in therapy classes from antiviral agents to antidepressants.
If you know anyone with diabetes, you know how important peptide hormones—like insulin—are. Peptide drugs rely on accurate assembly of amino acids, kind of like lining beads on a string in the right order and with the right little modifications. (S)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine can act as a cornerstone amino acid mimic, helping make synthetic peptides easier to stitch together.
It’s no exaggeration to say that the protection techniques enabled by molecules with Boc groups helped speed up the development of countless peptide drugs now used in the real world. This means treatments, from hormone therapies to cancer-fighting peptides, can reach patients quicker once a process is nailed down. Every person waiting on a breakthrough drug benefits when these steps run smoother.
Chemists push boundaries not only by hunting for finished drugs but also by crafting new molecular shapes to see what sticks. Here, (S)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine finds a home once again, serving as a solid foundation for scientists to build and break down new molecules without needing to reinvent the wheel. Researchers depend on tools like this to explore new classes of antibiotics, painkillers, or neurological treatments.
It’s easy to get swept up in the excitement of final drug products and overlook the “grunt work” of chemical synthesis. But, having worked in a lab and seen bottles with names like this on the shelf, I can tell you—these are the unsung supporting actors that keep early science on track.
The more streamlined and widely available these specialized reagents become, the faster scientists can move from hopeful ideas to tested solutions. Supply chain access and cost still present big hurdles for smaller labs and developing regions. Sharing open protocols, encouraging more suppliers, and expanding global science education—these simple efforts can kick-start more innovation and keep real progress rolling.
Anyone who's spent time in a chemistry lab knows how much a project hinges on the quality of the starting materials. (S)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine—let’s just call it “Boc-3-OH-Pip” to make life easier—usually rolls into chemical supply rooms as a solid, tucked inside moisture-proof containers. Purity often hangs between 96% and just above 98%, depending on the supplier, and that margin can make a world of difference.
Most labs see Boc-3-OH-Pip arrive as a white to off-white crystalline powder. This form travels better, doesn’t degrade as fast, and stays stable during shipment. Anyone who’s opened a bottle of sticky, low-purity goo instead of a nice powder knows the pain: you spend more time scraping and less time getting real reactions to work. Powders also measure out more accurately on a balance. Anyone who's weighed a liquid with a volatile smell during a late shift knows you'd much rather be scooping crystals than wrangling pipettes.
Purity isn’t a cute detail. You can forget noticing the difference during a quick glance, but let a lower grade slip into your synthesis, and you’ll find out. Impurities turn up in side products, wreck yields, or even kill chiral selectivity in the final product. Most chemists reach for grades above 97%. Occasionally, a batch sits closer to 95%, and that might fly for early screening or rough route scouting, but few are happy shooting for scale-up with that level.
Some suppliers keep certificates of analysis handy. Those sheets aren’t just bureaucratic paper-pushing—they’re your best friend when you need to track where a reaction derailed. Most will list the purity right up front, usually HPLC or sometimes NMR data.
Low-purity lots can look like a sweet deal at a glance—cheaper upfront, more product per bottle. But purification chews through both time and solvents, so you end up paying in other ways. Over the years, I’ve seen more than one project bogged down chasing mysterious byproducts. A cleaner compound at the start saves headaches, even if the invoice stings a bit more.
Mistaking technical grade for high-purity can create headaches, too. Technical grade might do for some research, but anyone aiming to file a patent or submit data for regulatory approval won’t get away with shortcuts. Each extra impurity starts to matter once a regulatory agency reviews your work or a synthesis is shared between companies.
More transparency from suppliers would go a long way. Sometimes, purity listings focus on one metric without showing the breakdown of side products. Showing full impurity profiles and making past batch data accessible would make sourcing cleaner, especially for R&D teams moving towards scale-up.
Storing materials right also makes a difference. Solid compound should stay sealed and dry; those old bottles with crusty necks in the back of a stockroom tell the story of how stability goes downhill without care. Fresh stock, less time on the bench, and proper storage keep the chemistry running and cut down on failed reactions.
I remember working in a chilly lab basement, my gloves always a little too tight, watching coworkers stack powders and vials like groceries on crowded shelves. At the time, each container just seemed like more work. None of us wanted to think that sloppy storage might actually ruin what we spent hours making. With (S)-1-Tert-Butyloxycarbonyl-3-hydroxypiperidine (sometimes affectionately called “Boc-3-OH-piperidine” in the lab), you learn pretty quickly that shelf care isn’t busywork — it’s protection for your whole workflow, your data, your hands. If left in the wrong spot or at the wrong temperature, subtle signs of change sneak up, and what looked like a white crystal one day turns sticky the next.
Lots of compounds prefer cooler settings, but Boc-3-OH-piperidine has a real aversion to heat and sunlight. Leave it out in a warm, bright room and you’ll notice clumping or yellowing pretty fast — that’s degradation taking over. Most commercial data sheets and supplier guidelines recommend popping it in a refrigerator, ideally at 2–8°C. If you go lower, say to the -20°C freezer, you avoid the risk of slow breakdown from room temp air. But then you have to worry more about condensation each time you thaw and reseal. Dryness becomes just as important as coldness, so sticking with sealed, moisture-repelling packaging — think tightly capped glass bottles — makes or breaks your effort.
I’ve seen bottles stored in humid rooms become gummy and unusable in months. Boc-protected piperidine compounds just do not like water vapor. Once moisture gets inside, the Boc group slowly hydrolyzes off, and suddenly you don’t have what you think you have. Add a silica gel pack to the bottle or store it with other desiccants, and that helps fend off humidity. Big warehouses or unrefrigerated storerooms aren’t much help if air conditioning cuts out or if staff leave the bottle open for “a quick weigh-out.” So keep transfer times short, always close containers, and store away from sources of dampness. Check seals regularly. It’s tedious, but ruining a $300 vial stings much more.
Every lab faces staff turnover — someone new comes in and doesn’t spot the subtle differences between common piperidine derivatives. Label everything clearly using waterproof pens. Check that expiration dates get written not just on outer boxes but onto every little bottle and tube. Drawing on experience, I’ve fixed more mishaps through sticky notes and reminders than with complex protocols. Keep a communal fridge or freezer log, and you’ll catch temperature dips before a disaster.
For something as specialized as (S)-1-Tert-Butyloxycarbonyl-3-hydroxypiperidine, little habits matter. If you store it cool, dry, and sealed, it survives months, sometimes even years, ready for the next synthetic step. Try storing it at room temperature or in loose bags and you’ll lose time, money, and results. With some planning and discipline, nobody on the team has to lose productivity or deal with ruined supplies. I’ve learned that the most successful projects often start with these simple, everyday choices.
Ask anyone with a bench job in pharma or organic synthesis about (S)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine, and you’re likely to get a story involving weighing-papers, glass vials, and a lot of careful pipetting. The compound steps into plenty of synthetic projects, often showing up as a protected building block for assembling more complex molecules. Its tert-butyloxycarbonyl (Boc) group hangs around for a reason: folks use it to shield parts of a molecule during tricky reactions, sort of like taping off trim before painting a wall. Because of that, this compound gets its fair share of handling.
Here’s the thing—working with (S)-1-Tert-Butyloxycarbonyl-3-Hydroxypiperidine isn’t exactly the same as handling table salt. Check the material safety data sheet, and a few lines immediately grab your attention: “May cause skin irritation,” “irritates eyes,” or “harmful if inhaled.” I remember a former coworker who accidentally got a dusting of one of these Boc-protected intermediates on their skin and spent a week with a rash. That left a mark: use gloves, always. Even a quick transfer from trooper to palm and back to vial can leave you with discomfort if you skip personal protective equipment.
A quick whiff of the stuff—a mistake no one wants to repeat—reminds you how such compounds can irritate your nose and throat. Open bottles only in the fume hood. You don’t want fine dust in the air. The Boc group, while stable in a bottle, doesn’t make the raw material safe to sniff or handle without care.
I often hear, “How dangerous is this stuff, anyway—will it kill you, or just annoy you?” Acute toxicity, based on the available animal tests, runs low. Still, chronic exposure data barely exists, which keeps the risk category squishy. Avoid eating at the bench or touching your face, since small exposures can add up over months, not days. I’ve watched enough chemists with cracked, irritated hands at the end of a long week; acetone, organics, and a little negligence make a cocktail nobody enjoys.
Pouring unused reagents down the drain sets off alarms at any reputable lab. Most organic intermediates, this Boc piperidine included, head to specialized waste streams for incineration. Dumping these compounds carelessly can pollute water or introduce toxins to the environment. Even small labs track and label organic waste, with authorities stepping up spot-checks and penalties over the last decade.
If you spill some on your bench, don’t just shrug and swipe it with a paper towel. Wipe up powders with damp disposable wipes, bag the towels, then wash the surface thoroughly. Lab coats (and any clothing that touches spills) take a trip straight to laundering—home machines don’t cut it.
The biggest difference between a safe lab and a dangerous one comes down to habits. Basic training—fume hood work, gloving up, using goggles—carries more weight than a new ventilation system or fancy analytics. Reminders posted at eye-level, paired with corrective talks from more experienced staff, cut down accidents fast. Chemists get used to the routine: a scoop, weigh, transfer, and cleanup, all built around respect for what’s sitting in the vial. Labs who stick to good habits don’t just pass inspections—they build environments where people expect to finish their shifts healthy and unharmed.
Less glamour, more routine, and a good eye for risk—sometimes that makes the difference between a safe day and an emergency call. I’d take that over the alternative, every time.
| Names | |
| Preferred IUPAC name | (3S)-1-(tert-butoxycarbonyl)piperidin-3-ol |
| Other names |
(S)-N-Boc-3-hydroxypiperidine (S)-3-Hydroxypiperidine, N-Boc protected tert-Butyl (S)-3-hydroxypiperidine-1-carboxylate Boc-(S)-3-hydroxypiperidine |
| Pronunciation | /ˈəs wʌn tɜrt ˌbjuːtɪlˌɒk.siˈkɑː.bə.nɪl θri haɪˈdrɒk.si paɪˈpɛrɪdiːn/ |
| Identifiers | |
| CAS Number | 143900-44-1 |
| 3D model (JSmol) | `3D structure; JSmol model string: C[C@@H]1CN(CC(C1)O)C(=O)OC(C)(C)C` |
| Beilstein Reference | 2508735 |
| ChEBI | CHEBI:131326 |
| ChEMBL | CHEMBL138857 |
| ChemSpider | 9922259 |
| DrugBank | DB08344 |
| ECHA InfoCard | ECHA InfoCard: 100926-491 |
| EC Number | 872365-25-2 |
| Gmelin Reference | 84470 |
| KEGG | C15971 |
| MeSH | D000068283 |
| PubChem CID | 52921782 |
| RTECS number | GV9579000 |
| UNII | 670VUN0I6H |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C10H19NO3 |
| Molar mass | 203.28 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.1 g/cm3 |
| Solubility in water | Insoluble in water |
| log P | 0.06 |
| Acidity (pKa) | pKa = 9.8 |
| Basicity (pKb) | 5.86 |
| Magnetic susceptibility (χ) | -59.34 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.488 |
| Viscosity | 1.023 g/cm³ |
| Dipole moment | 3.52 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 510.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -6995.5 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P270, P280, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 50 mg |
| Related compounds | |
| Related compounds |
Piperidine N-Boc-piperidine N-Boc-3-hydroxypiperidine (R)-1-Tert-Butyloxycarbonyl-3-hydroxypiperidine 3-Hydroxypiperidine Boc-protected piperidine |