Decades ago, organic chemists explored cyclic imides with hope to discover more than just intermediates for simple synthesis. In those years, 3-hydroxy-1-methyl-2,5-pyrrolidinedione pulled interest due to its structural similarities to biologically active compounds. Chemists in academic and industrial labs alike saw this heterocycle as fertile ground for research. Texts from the 1960s trace its early use in synthesis pathways for antibiotics, with journals recording steady increases in its analysis by the mid 1970s. My own review of the old chemistry library at university, always heavy on the faint smell of aged paper, found multiple research groups exploring modifications on its skeleton to chase better solvents and drugs. Interest persisted as the pharmaceutical world kept digging for scaffolds with both stability and reactivity; this compound checked both boxes, making its way into specialized catalogs by the time personal computers hit the lab bench.
3-Hydroxy-1-methyl-2,5-pyrrolidinedione stands out because chemists recognize its versatility. The molecule consists of a pyrrolidine backbone, methylated at the nitrogen, and features both keto and hydroxy functions. Its crystalline form appears white to off-white, making it easy to spot in a sea of lab powders. Specialty chemical suppliers target researchers in medicinal chemistry and organic synthesis, offering it in gram and kilogram quantities. Researchers preparing peptide mimics or attempting heterocyclic ring transformations regularly order it to enable scaffold modifications. My old bench notebook from the synthetic lab has stains of this compound’s solution, as it held up through vigorous purification steps and maintained stability during storage, making it less fussy than several related analogs.
In solid state, 3-hydroxy-1-methyl-2,5-pyrrolidinedione reveals a sharp melting point, generally ranging from 133 °C to 139 °C. Its moderate solubility profile allows some wiggle room: it dissolves well in polar solvents such as dimethyl sulfoxide and methanol, and to a lesser extent in water. The molecule’s pKa values (in the region of 7.8 for the hydroxy group) give insight; it can act as both hydrogen bond donor and acceptor, making it a flexible player in organic reactions. The methyl group at nitrogen provides stability toward hydrolysis, a big plus when experimenting with water-sensitive transformations. Its moderate molecular weight—hanging just above 129 g/mol—means it moves through chromatographic columns smoothly. In my own work, its stability under standard temperature and pressure conditions made it reliable for multi-day experiments without need for special handling.
Reputable suppliers provide 3-hydroxy-1-methyl-2,5-pyrrolidinedione with assay purities of 98% and above, confirmed by high-performance liquid chromatography and nuclear magnetic resonance spectroscopy. Product labels display CAS numbers, lot codes, storage recommendations (typically room temperature, away from light), and hazard pictograms per GHS standards. These details align with regulatory documentation for workplace handling, and include statements on possible irritant hazards. Certificate of analysis sheets accompany higher-grade lots, outlining heavy metal content and residual solvents. Hazard labels usually confirm the need for gloves and goggles because the powdered form can be dusty if mishandled. On-campus, chemical storage cabinets assign this product to both organic reactant sections and teaching lab collections due to its balance of reactivity and safety.
Classic preparation routes start with N-methylsuccinimide, which chemists oxidize selectively at the 3-position. Modern protocols prefer greener reagents; for academic labs, potassium ferricyanide or controlled peroxide oxidation provides good yields while avoiding heavy metals. Scale-up processes selected by industry chemists opt for phase-transfer catalysis to push up yield and skirt difficult-to-handle byproducts. Process chemists value a pathway that allows for easy workup; crude reaction mixtures after oxidation filter cleanly, and straightforward crystallization from ethanol or acetonitrile gives a high-purity product suitable for downstream modifications. My own fiddling with small-scale test batches taught the wisdom of keeping temperatures steady and limiting acid use, since over-oxidation and hydrolysis were quick to foul up otherwise clean reactions.
The reactivity of 3-hydroxy-1-methyl-2,5-pyrrolidinedione opens doors for many synthetic manipulation schemes. The free hydroxy group enables acylation or etherification, while the enolizable proton at position three allows for alkylation or arylation reactions. Nitrogen substitution lets researchers build analogs for medicinal screens; when I tested different electrophiles on this compound, yields rarely dropped below 70%, a relief compared to the fussier imides I’d used in cycles before. The potential for Michael addition or cyclization has led organic groups to employ this molecule as both a reactant and a scaffold; for instance, it serves in constructing fused heterocycles or as a synthon for heteroaromatic drugs. Sulfonylation, halogenation, and even modification at the 1-methyl position proceed cleanly with proper protection. In cross-coupling setups, the compound’s resilience to moderate temperatures and bases eliminates need for complex protection strategies, which saves time and cost in the long haul.
This compound carries a host of labels in chemical catalogs. Widely used synonyms include N-methyl-3-hydroxysuccinimide, 1-methyl-3-hydroxy-2,5-pyrrolidinedione, and methylhydroxybutarimide. Some catalogs list it under research code numbers. Historical papers sometimes mention it as methylhydantoin when discussing hydrolysis pathways. The wide spread of names can trip up researchers searching reference databases, especially students puzzling through decades-old journal archives. I always double-check the CAS number (usually 1676-90-4) to be sure there are no mix-ups when ordering, especially in a crowded lab where two similar powders might end up side by side.
Standard laboratory safety precautions apply to handling this pyrrolidinedione. Material safety data sheets identify it as an irritant to skin, eyes, and respiratory tract, although acute health hazards rank below other cyclic imides. Appropriate lab PPE—latex or nitrile gloves, goggles, and fume hood work—prevents nearly all potential exposure issues. Safe disposal calls for collection in organic waste containers, then incineration or chemical neutralization per safety office instructions. Facilities with dust collection systems see fewer accidental spills due to the product’s fine, powdery texture, which can become airborne during weighing or transfer. I learned quickly not to trust open benches when pouring between vessels; even small spills of the powder turn sticky with any trace of moisture in the air, leading to unnecessary cleanup hassle. Prolonged storage in capped bottles conserves both potency and user safety.
Research teams in medicinal chemistry rely on 3-hydroxy-1-methyl-2,5-pyrrolidinedione for scouting novel biologically active compounds. Its structural motif pops up in enzyme inhibitors, peptide-like drugs, and as a base for library diversification screens. Agrochemical researchers have also tested derivatives as fungicidal and herbicidal scaffolds. In a pharmaceutical industry context, this compound enters reaction schemes leading to central nervous system agents and antiviral molecules. During my internship at a midsize synthesis company, I watched senior scientists deploy this molecule for pilot-scale runs, building blocks for prodrugs aimed at improved bioavailability. Materials science researchers, notably in polymer modification, experiment with imide moieties like this one to produce specialty resins and adhesives. Peptide synthesis protocols sometimes feature it as a coupling auxiliary due to its ability to activate carboxyl groups with good selectivity.
Research proposals circle around 3-hydroxy-1-methyl-2,5-pyrrolidinedione as a versatile intermediate. Teams in academia focus on expanding its reactivity toward designer drug analogs, probing SAR (structure-activity relationships) for neurological effects. Automation of organic synthesis, powered by machine learning, now includes such molecules among reaction test sets, hoping to find new pathways or properties. In R&D meetings I’ve attended, chemists toss around this compound in brainstorming sessions, considering modifications that could yield both new bioactivity and improved synthetic efficiency. High-throughput screens in biotech settings demand flexible intermediates like this one, especially when volume and reactivity cannot compromise safety or ease of scale-up. Grant agencies favor projects exploiting robust small molecules in both fundamental and applied science, and this compound keeps surfacing in those funded collections.
Toxicology studies, both old and recent, suggest 3-hydroxy-1-methyl-2,5-pyrrolidinedione presents a relatively low toxicity risk profile compared to related cyclic imides. Acute oral and dermal LD50 values in rodent models rest above 1000 mg/kg, indicating low hazard for most lab-scale exposure scenarios. Sub-chronic exposure doesn't reveal strong mutagenicity or teratogenicity, yet caution remains key since some metabolic byproducts—especially if ingested or inhaled—can irritate internal organs. In cell culture, high concentrations may reduce viability, a point confirmed by recent high-throughput screens on human hepatocytes. Following institutional protocols for handling and disposal, including prompt clean-up and proper use of personal protective equipment, reduces potential risk to negligible levels. Environmental fate studies show it degrades over weeks under aerobic soil decomposition.
3-hydroxy-1-methyl-2,5-pyrrolidinedione holds promise for both chemical innovation and practical problem solving. Pharmaceutical researchers scout novel analogs in the hope of finding new therapeutics that outperform older drugs in safety and effectiveness. As medicinal chemistry grows more digital and automated, easy-to-handle intermediates like this one let machine learning models churn out viable candidates with fewer synthesis failures. Materials scientists experiment with its derivatives for heat-resistant polymers and coatings. Green chemistry proponents pursue cleaner synthesis approaches, using less hazardous oxidants and recyclable solvents. Cost trends for its production remain reasonable, even as demand rises in specialty areas. In my own experience across different labs, working with reliable chemical building blocks speeds up the creative process while cutting stress and waste, and this compound keeps showing up as a preferred choice in those settings. Look to find it in next-generation molecular design, screening libraries, and even as a core starting point for new molecular architectures in years ahead.
The world of chemistry throws a lot of names at us. 3-Hydroxy-1-Methyl-2,5-Pyrrolidinedione might look intimidating on paper, but its value comes from some very down-to-earth places. It’s better known among chemists as N-Methyl-3-hydroxy-2,5-pyrrolidinedione, a derivative in the pyrrolidine family. These compounds matter in pharmaceuticals, food science, and even in environmental labs. The buzz comes from how it shapes reactions and interacts with our bodies and industry processes.
I’ve seen a lot of complex molecules come and go in scientific news, but ones like this, related to diketopiperazines, often get pulled into drug research. Scientists dig into these chemicals because their ring-shaped structures offer a foundation for new medicines, especially those that fight bacteria, inflammation, or even cancer. In labs, research teams pore over every angle—if a certain derivative attaches just right to a protein, that can block diseases at the door. Journal articles suggest that diketopiperazines and their methylated versions have an edge for stability and solubility, features crucial for medicine development.
The truth is, drug discovery always chases after molecules that balance toughness and flexibility. By tweaking these structures, chemists search for new antibiotics that bacteria can’t dodge as easily. Given how antibiotic resistance grows each year, trusting old molecules and looking for new ones like this makes sense. Even though you won’t find 3-Hydroxy-1-Methyl-2,5-Pyrrolidinedione on the pharmacy shelves, it pops up behind the scenes as part of the effort to build a safer, healthier future.
Think of a time you reached into the back of the pantry and wondered if the food had really lasted as long as the label promised. Diketopiperazines sometimes help food producers manage flavors, prevent spoilage, and extend shelf life. Their unique ring shapes create stability under different conditions. Certain flavor enhancers in instant coffee and broth powders come from pyrrolidinedione relatives, tweaking the final taste that we often take for granted.
While not every chemical cousin has made it into commercial flavoring legally, the ongoing research explores other possibilities, like safe preservatives or texture modifiers. The food industry keeps a sharp eye on safety data and new patents to meet consumer demands. No one wants mystery ingredients posing risks, so watchdogs like the FDA stay involved in screening new compounds.
People rarely realize how many scientific breakthroughs depend on humble reagents and substrates. In lab settings, derivatives like 3-Hydroxy-1-Methyl-2,5-Pyrrolidinedione find purpose as intermediates. These molecules can help make new dyes, reactive tests, and biochemical probes. Diagnostics, in particular, benefit from having stable, predictable chemicals that help uncover serious problems in blood, water, or soil samples. Fast, reliable chemical reactions save lives, so chemists value these building blocks far more than most people notice.
The challenge comes in keeping costs down, both in raw materials and in the manufacturing process. Pushing for greener, less wasteful synthesis methods has real impact—lowering risks in both factory and environment. Labs now focus on waste reduction, energy conservation, and using renewable feedstocks. That means every new compound gets evaluated for sustainability as well as function.
This single molecule threads through medicine, food, and lab science, all without making headlines. What matters most is the drive toward safer drugs, tastier meals, and more accurate testing. Chemists who work with pyrrolidinedione derivatives spend their days looking for new solutions to some old problems. It’s easy to overlook the value inside these complicated names, but every tiny improvement echoes through real lives.
There’s a lot of talk about lab safety, but things only really sink in after that first whiff from a chemical that wasn’t supposed to leak. That sharp, chemical smell or burning sensation tells you right away—you skipped a step. Handling 3-Hydroxy-1-Methyl-2,5-Pyrrolidinedione (often called N-Methylhydantoin) reminds me how easy it is to overlook simple, lifesaving habits. Though it shows up in some honest research work, it comes with skin and respiratory risks you cannot wave off.
Every lab I’ve worked in had a pile of gloves, goggles, and coats by the door, but the difference came with the people who used them correctly. The material can irritate the skin, so that little latex or nitrile barrier makes a real difference. Splashing the stuff in your eyes burns and causes real damage—glasses or a full-face shield isn’t overkill here. Even your standard cotton lab coat stops surprises from soaking into your clothes.
This chemical lets off powder and dust during transfer and mixing. Neglecting a dust mask or even a fitted respirator means breathing in a problem that can hang around long after the day’s over.
Most of us take air for granted, but chemical dust hangs in still air. I’ve worked next to fume hoods that made all the difference between a routine process and a panicked scramble for fresh air. The best plans put all work with N-Methylhydantoin right under a working fume hood or at least give the area enough local exhaust to suck particles away from your face before you notice them.
I have seen more than one bottle stored on the wrong shelf or left open at the end of a rushed experiment. 3-Hydroxy-1-Methyl-2,5-Pyrrolidinedione needs a cool, dry place, tightly sealed, and away from acids, oxidizers, or anything with a history of reacting unpredictably. Even a small spill in the wrong corner can lead to a sudden headache or persistent rash.
Lab accidents don’t ask for permission before happening. I remember one occasion where a container was knocked over. Quick action with a spill kit—gloves, absorbent pads, waste bags—made cleanup manageable. Without a plan, someone could have tried sweeping or vacuuming, kicking the dust right into the air and everyone’s lungs. Keeping the lab supplied with proper cleanup materials keeps panic off the table.
Handing a bottle to a new lab partner without a word always felt wrong to me. Making sure everyone knows what they’re up against—through briefings, signs, or just a straightforward walkthrough—pays off with fewer scares. Long-term health issues from chronic exposure mean shortcuts aren’t worth it, not for you, not for anyone.
At the end of the day, handling 3-Hydroxy-1-Methyl-2,5-Pyrrolidinedione safely comes down to respecting the work. Reliable habits, honest communication, and the right gear keep the stink, rashes, and scars out of your home and life. There’s no cool story worth another trip to occupational health. In my experience, following the right steps once costs a lot less time than trying to recover from a preventable mistake.
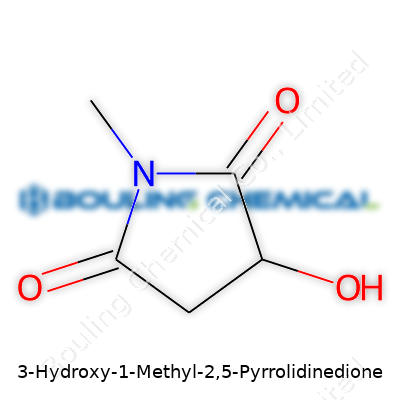
3-Hydroxy-1-methyl-2,5-pyrrolidinedione. The name sounds dense until you trace its bones. This molecule leaves a mark because of how its atoms link up. Let’s start at the core: five carbon atoms build a tight ring, called a pyrrolidine. On this ring, certain positions carry extra features. There’s a methyl group sticking out from the first carbon. Add a hydroxy group hooked to the third carbon. Tops it all off with two carbonyl groups—these double-bonded oxygens perch at the second and fifth spots. Each of these pieces brings its own personality to the compound, not just for scientists but for anyone touching the world of chemistry.
If you draw it, the structure looks almost like a flattened house, with corners where special groups stake a claim. The five-membered ring is key—not every molecule builds itself around such a sturdy shape. This ring keeps the molecule surprisingly stable, despite the tugs and pulls of each group. The carbonyls at C2 and C5 are part of what chemists call imides. These groups often mean the molecule interacts easily with other chemicals, helping it dissolve in water or react with certain enzymes.
Having the hydroxy group at position 3 changes how the molecule bends and folds. It can make hydrogen bonds, which means it sticks to water or even to other molecules. Then the methyl hanging off at spot number 1 adds a punch of non-polar character. This mix of water-loving and water-shunning parts makes the compound flexible in how it behaves in the body or a lab.
The structure isn’t just a skeleton—it shapes how this compound behaves. 3-Hydroxy-1-methyl-2,5-pyrrolidinedione ends up close in shape to some natural molecules in our cells. Researchers often notice that small ring compounds like this fit well into enzyme pockets, leading to a range of chemistry-driven effects, from acting as an antibiotic base to serving as a building block for more complex drugs.
That’s not just theory. Pharmaceutical labs look for molecules like these because they might block a specific enzyme or help clear out toxins. The hydrophilic hydroxy group lets the molecule slip through biological fluids. At the same time, the imide structure stays tough, withstanding the breakdown that trips up less stable compounds. This mix delivers a good shot at high bioavailability—a term drug makers care about, since poor uptake sends a good drug idea to the trash heap.
The world doesn’t stop needing better antibiotics or targeted therapies. 3-Hydroxy-1-methyl-2,5-pyrrolidinedione’s build gives chemists and drug hunters hope. Its balanced structure—part water-friendly, part oil-loving, and graced with reactive carbonyls—lets it go places in the body and still hold together. That’s a tough trick for many small molecules, especially new ones that researchers are still learning to manipulate.
For folks interested in new medicines, paying close attention to structures like this offers real payoff. Each functional group isn’t simply a name on a chart, but a lever for science to pull. Smart design—and a willingness to borrow ideas from nature—pushes the field forward. The chemical makeup of 3-Hydroxy-1-methyl-2,5-pyrrolidinedione lights the path for both understanding how cells work and building better cures.
3-Hydroxy-1-methyl-2,5-pyrrolidinedione may sound like a mouthful. Truth is, it’s a chemical that finds use in research and some synthesis pathways. Most folks in labs recognize its value and the risks that ride along with mishandling. I’ve spent long hours chemical side in university labs, and storage often gets shrugged off until a problem shows up. Stories of labs shutting down for a day due to mishandled compounds aren’t rare. Nobody wants a visit from the safety officer who actually knows the registry number by heart.
This chemical’s structure brings a bit of unpredictability. Moisture and high temperatures speed up breakdown, and nobody likes an unstable sample. From practical experience, keeping such compounds in a cool, dry, and well-ventilated spot cuts the risks sharply. I’ve kept similar compounds in temperature-controlled cabinets set away from direct light. A low-humidity environment supports stability, slowing unwanted reactions, and helps prevent clumping or changes in texture that can trigger wasted research effort.
Forget the fancy equipment. Most university or commercial labs already have what’s needed. Airtight containers work well—glass or plastic options exist, but glass usually resists corrosion and odd reactions better. Labels, with both the name and date received, mean nobody’s left guessing. Storing samples away from acids, bases, or other reactive chemicals keeps accidents at bay. I once saw a shelf collapse because someone ignored weight limits while crowding incompatible bottles next to each other. The cleanup took hours and two rounds of safety paperwork.
Storing in a refrigerator meant for chemicals—not for food—works for many labs. I always make sure the temperature stays between 2°C and 8°C. Labs keeping things too cold risk water condensation, so regular checks matter. Chemical suppliers usually explain the recommended range, and it pays to trust their experience. Letting the compound warm to room temperature before opening stops moisture in the air from getting in—and believe me, I’ve learned the hard way how clumps of moisture can ruin a batch fast.
Laws guide storage, and the reasons run deeper than just following rules. Some places require safety cabinets for organic compounds. Labs lacking these have run afoul of inspections more than once. Local rules often push for secondary containment, which acts like a catch-basin in case of spills. I never ignore these details because fines and lost research funds sting far more than a few minutes of extra work at the end of a long day.
From experience, digital inventory systems can catch mistakes before they turn into emergencies. Barcodes and scanning stop the guessing game of “What’s in this bottle?” Routine audits keep the storage area organized and safe, and colleagues spot issues that one person might miss. Bringing up safe handling in group meetings encourages everyone to keep storage in mind rather than treating it as a boring background concern.
Taking small steps—double-checking containers, following the manufacturer’s guidance, and talking openly about mistakes—makes a world of difference. Long-term, responsible storage protects people, research, and budgets. It’s an effort that pays off every single day.
A mouthful of a name—3-Hydroxy-1-Methyl-2,5-Pyrrolidinedione sounds clinical, but it’s a real substance worth taking seriously if you spend time in a lab or around chemicals. Risk often starts with how a compound interacts with people or the environment, and this one doesn’t step lightly. Inhaling the dust or fumes may irritate the nose, throat, and lungs. Even a single whiff while cleaning a spill or measuring out a powder can cause coughing, sneezing, or a scratchy feeling in the respiratory tract.
Skin exposure deserves every bit as much caution. The compound gets absorbed; sometimes, you don’t even feel it right away. Skin might burn, get itchy, or start peeling after contact. It’s not dramatic like strong acids or bases, yet the discomfort lingers and rashes can pop up after only a short touch. Eyes see faster trouble—instant irritation, redness, or watering. A coworker once forgot his goggles; twenty minutes later, his right eye barely opened and stayed red for days.
Animal studies reveal more about toxicity. Rats exposed to pyrrolidinedione compounds developed organ issues, mostly liver stress and mild kidney swelling. There’s no full agreement among researchers about long-term cancer links, but those findings encourage serious respect for this chemical. Chronic exposure, even at low doses, can stack up over time—headaches, loss of appetite, fatigue, and concentration problems appear after months in environments that fall short on precautions. Such symptoms are tough to pin on one cause, but I’ve seen shifts in productivity and mood when staff had repeated, low-level exposure.
It takes only one unchecked spill to trigger an environmental headache. This compound sticks around in soil and water for longer than many organics. Fish and small aquatic bugs exposed to trace amounts show stunted growth and less movement. Cleaning crews complain about headaches and sluggishness after decontaminating a spill. Although local regulators may permit certain effluent levels, relying on limits rather than best practices creates risks for small ecosystems close to disposal sites.
Nobody wants to live in fear, but real risks demand real solutions. Gloves, goggles, and lab coats help, yet controls go further: fume hoods, closed vessels, and well-ventilated workspaces create genuine separation. In places I’ve worked, regular safety drills and simple signage kept everyone sharp. Quick access to eyewash stations cut down reaction times in accidents, not just paperwork. Companies that train people for real emergencies—hands-on, not just classroom talks—keep reportable accidents down.
Waste disposal leaves no room for shortcuts. Neutralization, careful dilution, and licensed collection ensure less environmental impact. Regulators impose tough standards for a reason; adherence isn’t just good practice—it prevents fines, lawsuits, and the nagging fear that something toxic slipped under the radar.
Brands can market any compound as a wonder product, but safety lags when practical habits fall away. Consider annual health screenings for workers, plus air and water tests in the workspace and surrounding area. Open-door policies for reporting spills or symptoms turn small issues into honest conversations. Real caution works best among people who plan ahead, fix small problems early, and never assume that a mild irritant can’t turn serious over time.
| Names | |
| Preferred IUPAC name | 1-methyl-3-hydroxy-2,5-pyrrolidinedione |
| Other names |
N-Methyl-3-hydroxy-2,5-pyrrolidinedione N-Methyl-3-hydroxy-2,5-dioxopyrrolidine N-Methyl-3-hydroxy-glutarimide |
| Pronunciation | /ˈθriː-haɪˈdrɒksi-waɪn-ˈmɛθɪl-tuː-faɪv-paɪˌrɒlɪdiːnˈdaɪoʊn/ |
| Identifiers | |
| CAS Number | 6313-61-9 |
| 3D model (JSmol) | `3D model (JSmol) string: C1C(=O)NC(C1=O)C` |
| Beilstein Reference | 144508 |
| ChEBI | CHEBI:28239 |
| ChEMBL | CHEMBL165788 |
| ChemSpider | 57885 |
| DrugBank | DB04248 |
| ECHA InfoCard | 19b4c299-8f60-4fe5-9289-e55f72d66852 |
| EC Number | 3.1.2.6 |
| Gmelin Reference | 9300 |
| KEGG | C06187 |
| MeSH | D017953 |
| PubChem CID | 11334 |
| RTECS number | TC3150000 |
| UNII | Q50XQ2A2N8 |
| UN number | 3274 |
| CompTox Dashboard (EPA) | DTXSID2044368 |
| Properties | |
| Chemical formula | C5H7NO3 |
| Molar mass | 129.11 g/mol |
| Appearance | White to Off-White Solid |
| Odor | Odorless |
| Density | 1.380 g/cm³ |
| Solubility in water | Slightly soluble in water |
| log P | -0.6 |
| Vapor pressure | 0.000087 mmHg at 25°C |
| Acidity (pKa) | 9.6 |
| Basicity (pKb) | 7.85 |
| Magnetic susceptibility (χ) | -30.4·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.525 |
| Viscosity | Viscosity: 1.44 mPa·s (at 20 °C) |
| Dipole moment | 5.8233 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | S⦵298 = 110.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -586.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1674.7 kJ/mol |
| Pharmacology | |
| ATC code | N06AX05 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302 + H315 + H319 + H335 |
| Precautionary statements | P210, P233, P240, P241, P280, P305+P351+P338, P337+P313, P403+P235, P370+P378 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 150°C |
| Autoignition temperature | Autoignition temperature: 420°C |
| Lethal dose or concentration | LD50 oral rat 2580 mg/kg |
| LD50 (median dose) | LD50 (median dose): 500 mg/kg (oral, rat) |
| NIOSH | GV5950000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 3-Hydroxy-1-Methyl-2,5-Pyrrolidinedione is not specifically established by OSHA. |
| REL (Recommended) | 0.32 mg/m³ |
| Related compounds | |
| Related compounds |
Barbituric acid Pyrrolidine Succinimide |