Scientists in the field of organic chemistry started working with dibenzothiophene derivatives decades ago. Their early work centered on isolating sulfur-containing heterocycles from crude oil and coals. Interest grew in creating new compounds with enhanced reactivity and improved electronic effects for chemical synthesis, pharmaceuticals, and materials science. The addition of fluoro groups to aromatic systems opened up fresh tracks in modifying molecular stability and performance, especially as researchers figured out the powerful effects that halogens granted for pharmaceutical lead compounds. Ethoxy substitution expanded the range for interpolation of polarity and lipophilicity. These layered steps led to the realization of molecules like 3-Ethoxy-4,6-Difluoro-Dibenzothiophene. Each step in this history responded directly to industrial and research needs, which fueled evolution from base lignin and coal-tar products to today’s precision organic chemicals. The continued demand for advanced intermediates in electronics, drug science, and agrochemical innovation keeps progress moving forward.
3-Ethoxy-4,6-Difluoro-Dibenzothiophene has become a valued specialty chemical. Its dibenzothiophene skeleton offers chemical toughness and good compatibility with aromatic systems. The ethoxy group increases solubility in a range of organic solvents, while the two fluorine atoms modify the reactivity and can influence molecular recognition in pharmaceutical or materials applications. Labs and factories typically purchase this compound as a crystalline solid in tightly sealed packaging to prevent moisture uptake or degradation. Accurate labeling information supports handling and regulatory tracking. Producers invest in precision synthesis to guarantee the right substitution pattern, knowing that any error in the position of the ethoxy or fluoro substituents can throw off desired electronic properties and limit downstream utility.
This compound carries a molecular weight generally around 274 g/mol, reflecting the high atomic weight of sulfur and the presence of two fluorines. Its white to off-white powder form reflects high purity and the absence of colored impurities that result from side reactions or degradation. As with other dibenzothiophenes, it melts well above room temperature, confirming robust crystal lattice energy. Chemical resistance stands out: the arene system resists hydrolysis, and the fluorinated positions hold up against electrophilic attack, allowing for selective activation at other sites if needed. It tends not to dissolve in water but performs well in solvents like dichloromethane, chloroform, or THF, which matters in both reaction design and purification.
Manufacturers assign precise codes and lot numbers to shipments, supporting full traceability for research or regulatory requests. Chemical purity levels of 98% or higher serve as the standard, as residues from oxidation, hydrolysis, or incomplete reaction can create difficulties in downstream synthesis steps. Storage instructions emphasize dry, cool conditions away from reactive agents. Safety labels list both chemical and toxicological data, including handling precautions for skin and eye contact, as well as respiratory advice, since dust can irritate mucosal membranes. For industrial users, safety data sheets describe what to do in case of accidental exposure or spillage, illustrating the focus on continuing operator safety and environmental compliance.
Chemists favor multi-step procedures to get the substitution pattern just right on the dibenzothiophene core. Starting from commercially available dibenzothiophene, they introduce the first fluorine through electrophilic aromatic substitution, taking care to control temperature and reactant ratios for selectivity. Protection-deprotection sequences sometimes help navigate the multiple activation sites on the aromatic rings. The ethoxy group is typically attached by nucleophilic substitution, bringing in ethoxide with careful control to avoid over-ethoxylation or side reactions, especially since the fluorines can deactivate some ring positions. The route relies on in-depth understanding of aromatic chemistry, fluorine’s electronic effects, and sulfur’s polarizability. High-performance liquid chromatography and NMR spectroscopy confirm that each batch meets identity and purity standards, so products work as designed in research labs or manufacturing plants.
Chemists spend years developing reliable ways to modify dibenzothiophenes. The fluoro substituents in 3-Ethoxy-4,6-Difluoro-Dibenzothiophene are electron-withdrawing and resist further activation, but neighboring positions can accept electrophilic substitutions under certain conditions. The ethoxy group operates as a leaving group in more aggressive reactions and can be swapped for other alkoxy or functional groups in tailored syntheses. Cross-coupling reactions, like Suzuki or Buchwald-Hartwig protocols, thrive on these core structures, allowing the installation of aryls, amines, or even more complex motifs. In materials chemistry, these modifications help tune the optical properties, while in pharma, slight changes dramatically shift bioactivity. Such functional versatility makes this molecule a preferred intermediate, even if synthesis takes several steps and close monitoring.
Depending on the supplier, research article, or industry, the same molecule might show up as 3-Ethoxy-4,6-Difluorodibenzo[b,d]thiophene, or even using codes that reflect cataloging systems for chemical libraries. People used to working in medicinal chemistry often reference standard nomenclature from IUPAC to avoid confusion, though lab slang or project-specific codes pop up for quick communication. Awareness of these alternate names proves useful when searching literature databases or ordering from global suppliers, since small naming differences can send users down the wrong path, wasting time and resources.
Everyone working with sulfur heterocycles knows to take precautions. 3-Ethoxy-4,6-Difluoro-Dibenzothiophene doesn’t carry the same risks as organomercury or nitroaromatics, but flour-dry powders invite inhalation, and contact with organic solvents always means gloves and goggles belong in use. Fume hood work prevents accidental inhalation, especially when scaling up reactions or purifying by column chromatography. All waste streams must be collected for proper disposal or solvent recovery, since environmental regulations increasingly examine impact from specialty chemicals. Worker health monitoring extends to periodic medical check-ups for those in direct contact for lengthy times, while digital labeling streamlines audit and safety recall if necessary.
Use cases for 3-Ethoxy-4,6-Difluoro-Dibenzothiophene reach into multiple industries. Medicinal chemists reach for it as a scaffold in early-stage drug discovery. The combination of ethoxy and fluoro functionalities offers a starting point for exploring enzyme inhibition or receptor modulation. Functional dyes and organic semiconductors draw on the electrical effects of halogen substitution and the durability of the dibenzothiophene backbone. Agrochemicals often rely on sulfur heterocycles for improved environmental stability as well as bioactivity against pests or weeds. Whether it’s a pilot plant or a commercial pharmaceutical synthesis, operators value the reproducibility and handling ease of this compound, leveraging its clean aromatic transitions and downstream convertibility.
Chemical research with dibenzothiophenes pushes innovation in both synthetic methods and final product applications. Research groups constantly search for greener reaction conditions, looking to truncate multistep syntheses or cut down on toxic solvents. Machine learning and data mining approaches surface promising new analogs, where computational predictions look for tweaks in side chain length or substituent pattern for improved performance. Patents describe expanded uses in materials science, including organic solar cells and light-emitting diodes; in life science, research articles chart kinase inhibitors or fungicides based on such backbones. Open databases and academic-industrial partnerships boost the pace of discovery, ensuring this class of compounds stays relevant for new generations of chemists.
Every new chemical destined for commercial or pharma use undergoes a battery of toxicity tests. The sulfur atom in dibenzothiophenes can metabolize to sulfoxides in the body, prompting studies on liver enzyme interaction and downstream excretion. Fluorinated arenes typically pass through organisms unchanged, but metabolic stability means scientists double-check for bioaccumulation risks. Animal studies focus on high-dose acute toxicity, organ-specific injury, as well as possible mutagenicity. Labs also look at chronic low-level exposure, since workers might handle the compound over years. Environmental studies target persistence in soil and water, monitoring for breakdown products that might upset local ecosystems. These efforts combine to generate clear usage guidelines and help manufacturers optimize industrial hygiene.
Researchers already see new demands for molecules like 3-Ethoxy-4,6-Difluoro-Dibenzothiophene. Pharmaceutical companies push to find non-opioid painkillers, anti-infectives, and biodegradable insecticides, all of which turn to novel aromatic backbones for solutions. The ongoing revolution in organic electronics means OLEDs, OFETs, and organic PV cells increasingly call on precise organic frameworks, with aromatic fluorination offering large leaps in performance and stability. Sustainability regulations pressure industry to redesign syntheses for less waste, higher atom economy, or recovery of scarce elements like fluorine. Training the next generation of chemists to synthesize, analyze, and deploy these molecules safely and efficiently stands as an ongoing, necessary challenge.
Every block in the construction of modern technology starts with raw ingredients. 3-Ethoxy-4,6-difluoro-dibenzothiophene sits among these raw ingredients, shaping products that touch daily life without most folks ever seeing its name on a label. This molecule, rooted in the dibenzothiophene family, stands out in labs focused on fine-tuning specialty chemicals. You’ll find it woven into the story of pharmaceutical breakthrough, advanced materials, and clean energy innovation. These connections aren’t just interesting side notes. They show how substance design feeds the next wave of science.
Medicines rarely come straight from nature. Chemists bridge the gap with smart tweaks to molecular backbones. 3-Ethoxy-4,6-difluoro-dibenzothiophene gives researchers a tool for building novel drug candidates. Fluorine in the mix can transform how a molecule behaves in the body. It can help strengthen targets, resist metabolic breakdown, or reduce unwanted effects. In large pharmaceutical companies, this compound’s structure gets attention during the early stages of drug design. My own experience working alongside synthetic chemists taught me how the addition of fluorinated groups can lift a drug’s performance, leading to new therapies tackling difficult diseases.
Beyond medicine, this chemical shows up in the hunt for stronger, lighter, and smarter materials. Organic electronics—a space that promises flexible displays and plastic solar panels—leans on molecules like 3-ethoxy-4,6-difluoro-dibenzothiophene. The structure lets engineers fine-tune conductivity and stability. The shift to organic, carbon-based materials in electronics happened because these compounds could offer both efficiency and shape flexibility. Researchers across universities and startups bet on fluorinated dibenzothiophenes in prototypes for organic semiconductors, OLEDs, and next-generation batteries. These applications bring big challenges, but they also open the door to greener, more adaptable tech.
Every chemical brings questions about how it travels beyond the lab. Dibenzothiophenes in general have raised concerns over environmental persistence. These molecules stick around in soils and waters longer than some others. Companies sourcing or using such chemicals must take care about storage, waste streams, and air emissions. Regulations push toward safer handling and drive research into ways to break down or recycle these substances. The message in every research meeting: innovation can't outpace responsibility. Modern chemistry walks a tightrope—offering cleaner energy and medical miracles while making sure nature isn't paying the price down the line.
I’ve seen green chemistry principles move out of theory and into practice. Suppliers now give transparency on sourcing and purity of specialty reagents like 3-ethoxy-4,6-difluoro-dibenzothiophene. Labs set up closed-system reactions to capture byproducts. Regulators work more closely with companies, and the public asks smarter questions. The next wave of invention depends on this kind of accountability. Specialty chemicals matter, but so does the way society manages their journey from design table to end-of-life. Progress isn’t just the new molecule—it’s what people do, together, to make sure tomorrow’s breakthroughs are safe for everyone.
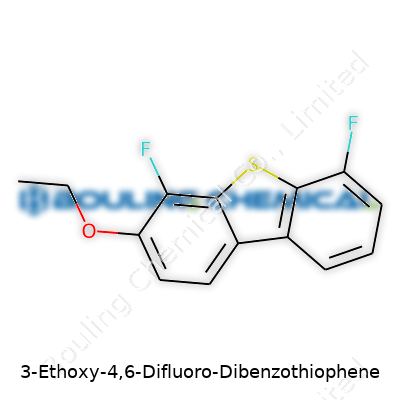
References:Chemistry gets real interesting when you look past the intimidating names and focus on the structure. Let’s talk about 3-Ethoxy-4,6-Difluoro-Dibenzothiophene. First, break the name apart. You see “dibenzothiophene”—one sulfur atom in the middle of two benzene rings—then, you pick up on the two fluorine atoms stuck at the 4 and 6 positions, and one ethoxy group at the 3 position. Knowing where various groups land lets you draw the molecule, which is how chemists crack these puzzles.
Dibenzothiophene, the base structure, rolls in with a formula of C12H8S. Slide the ethoxy group (–OCH2CH3) right in at position 3. Two fluorines come on at positions 4 and 6, booting out two hydrogens. That means for every halogen and ethoxy add-on, you have to subtract wherever a hydrogen gets replaced. Each substitution matters. Tally up what’s left: - Start with C12H8S.- Add C2H5O, which stands for ethoxy.- Add 2 F (for the two fluorines).- Remove two hydrogens to account for the ones swapped out by the fluorines.
Now do the math:Carbons: 12 + 2 = 14Hydrogens: 8 + 5 - 2 = 11Oxygen: 1Sulfur: 1Fluorines: 2So, the result: C14H11F2OS
Synthetic chemists, drug developers, and environmental scientists benefit when they get the correct formula. That formula flags the number of each atom, maps out reactivity, solubility, and explains what the compound might do in the body or the environment. Getting this wrong isn’t just a tiny error—it throws off every downstream calculation, from mass spectrometry predictions to reaction planning and regulatory paperwork.
I remember checking and rechecking chemical formulas during lab days. It always amazed me how one digit in the wrong place meant scrapping an afternoon’s worth of work. More importantly, mistakes like this can have bigger impacts, leading to errors in compliance, risk assessments, or even experimental safety.
Plenty of databases turn up with mismatched or outdated formulas for rare or specialty chemicals. Scientists have to trace the history, double-check substitutions, and look up chemical structures. Flipping through journals, patent databases, or chemical supplier catalogs, I’ve seen first-hand that formulas get misprinted or mistaken more than anyone likes to admit. The lesson: don’t trust a single source, always check the structure.
Teams should share structure files or hand-drawn diagrams during project work, not just names and formulas. Moving past just memorizing formulas, drawing out the molecule makes errors stand out. Using digital molecular editors or even hand sketches can pierce through confusion. For rare or new molecules, publish the drawn structure alongside the formula, so researchers down the line get clarity and greater accuracy.
Skepticism and careful cross-checking make great partners. No matter how obscure the compound, science moves forward by comparing sources, asking skeptical questions, and always putting clarity ahead of speed.
Anybody who’s spent time in a lab knows that no two chemicals act the same over time. Drawers and shelves full of strange names, but each one comes with its own rulebook. 3-Ethoxy-4,6-Difluoro-Dibenzothiophene has become more common in research labs, especially in pharmaceutical and organic electronics work. It might not be a household name, but this compound can create headaches fast if you fail to store it right. Company SOPs and chemical supply labels tell a story hard-won by people who have cleaned up shattered vials and seen what moisture or sunlight does to a carefully manufactured batch.
Like a lot of aromatic heterocycles, 3-Ethoxy-4,6-Difluoro-Dibenzothiophene starts to degrade if heat or UV sneaks in. I learned that the hard way years ago, tracking down yield losses in what seemed like a solid synthetic route—a batch that had darkened just enough from a too-warm storage cabinet. Keep this compound sealed tightly in an amber bottle, and tuck it away in a cool, dry space, ideally between 2°C and 8°C. Chemical refrigerators aren’t just for biology work; they shield organic molecules like this from temperature spiking every time a door swings open.
If moisture finds its way in because the container isn’t tight or the cap material reacts, hydrolysis follows. That means breakdown, impurities, and wasted money. The more fluorinated or ethoxylated the molecule, the more sensitive it can be—at least, that’s what colleagues in chemical safety always warn. My own rule after one near-miss: silica gel packets in storage drawers as a backup line of defense.
Some might wonder why so much concern over a bottle of powder or crystals. For one thing, research budgets rarely stretch to lost batches. More critical than lost cash, though, is what impurities do to results. Many organic syntheses depend on keeping starting materials pure. Add in a batch contaminated by oxidation or moisture, and suddenly reactions stall or give unexpected products. At scale—think pilot plant or contract manufacturer—a small slip on storage multiplies into big dollar losses and possibly ruined downstream processes.
Every regulatory guideline, whether from OSHA or REACH, lines up with the same theme: treat chemicals as investments worth protecting. It doesn’t have to mean a vault—just taking small steps to know what can go wrong. Simple things like double-checking container integrity on arrival, labeling containers with the open date, and keeping incompatible compounds apart pay off every time. Over the years, watching how one careless labeling error led to the wrong substance in the wrong solvent has taught most chemists in industry to never get complacent.
Training new researchers, I always stressed that the storage protocol isn’t bureaucratic red tape—it’s insurance. As soon as a bottle goes unsealed, decay can start if air or spores get in. A lot of newer synthetic compounds have unknown quirks, so erring on the safe side by using desiccators and cold storage isn’t overkill. If you ever hear a chemist groan over lost time and ruined compounds, odds are good they’ve dealt with an avoidable storage problem. Protecting expensive, specialty chemicals like 3-Ethoxy-4,6-Difluoro-Dibenzothiophene isn’t just good science—it’s respect for everyone’s time and effort.
I’ve learned that accountability works best when it gets built into daily routines. Posting laminated storage guides in every stockroom, color-coding storage for fast visual checks, and enforcing a quick end-of-week inventory all help. Mistakes shrink when teams start owning the chemical inventory, not just managing it. Teaching the chemistry behind storage—why moisture or heat matters for something specific—sticks longer than just repeating "keep in a cool, dry place.”
Every so often, an unfamiliar compound name makes its way into headlines or safety reports. 3-Ethoxy-4,6-difluoro-dibenzothiophene falls into that camp for most people outside the science world. It turns up in certain chemical syntheses and sometimes in pharmaceutical research, thanks to its structure with two fluorine atoms, an ethoxy group, and a sulfur-containing ring. That's not enough to say it's dangerous, but it does mean the right people ought to keep tabs on what it can do.
Fluorinated compounds often come with strings attached. Some stick around in the environment and do not break down easily. That matters because it raises questions about what happens if they leak or spill. Dibenzothiophene derivatives, in particular, have shown persistence in soil and water. The more persistent a compound, the bigger the chance it gets into living things.
At the same time, not all fluorinated dibenzothiophenes work the same way in the body. The parent structure has shown mild toxicity to aquatic life, but specifics for 3-ethoxy-4,6-difluoro-dibenzothiophene are sparse. No large-scale toxicology reports have surfaced, which leaves people relying on structural clues and old data on related chemicals. These reports show that similar compounds can irritate skin and eyes, and breathing their vapors could bother airways. Long-term concerns usually focus on bioaccumulation and what that means for animals in the food chain.
In laboratory work, not every chemical gets the same level of caution. The ones with long names and a whiff of sulfur often win extra respect. Years in research taught me that gloves and fume hoods aren't overkill, especially with compounds where the risk isn’t nailed down. I've seen too many reports where a new solvent or intermediate caused unexpected issues, from simple rashes to more serious environmental hiccups. The uncertainty matters, especially when the safety data sheet just says, “No data available.”
Messing around with complex aromatic compounds in a poorly ventilated space can lead to headaches or throat problems. Mislabeling or poor storage invites spills, and cleanup becomes someone else's job. People are right to wonder if new chemicals may linger in water or break down into something nastier. The history of chemical safety is full of regrets from ignoring these signs.
Regulators do not usually list 3-ethoxy-4,6-difluoro-dibenzothiophene by name. That is both good and bad. It means it hasn't caused big public health scares. It also means anyone using it professionally needs to step up personal safety and not assume the material is harmless. Standard rules call for gloves, goggles, and a way to vent vapors.
Better testing would help. Companies ought to run real-world studies on breakdown products, aquatic life exposure, and chronic inhalation effects. Open databases like PubChem have only sketchy information, so sharing new results benefits everyone. If the compound turns up in consumer-facing products, more transparency would go a long way.
The biggest step: Don’t wait for big problems before setting ground rules. Science moves fast, so safety rules need to keep up—not just after an accident, but in the regular day-to-day grind of the lab.
Anyone dealing with research or specialty chemicals sooner or later faces the challenging task of sourcing rare compounds. 3-Ethoxy-4,6-Difluoro-Dibenzothiophene sits squarely in that category. From personal experience in a college lab, the toughest times usually arrived while tracking down intermediates nobody kept in bulk. Instead of a quick catalog order, phone calls and email exchanges stretched out for weeks.
This particular compound pops up in medicinal chemistry and advanced materials work. Most graduate students and industry chemists look for it through specialist suppliers with a focus on custom synthesis or small-scale production. Familiar names in the space include chemical distributors like TCI, Sigma-Aldrich, and Alfa Aesar, but availability for rare molecules jumps around. Sometimes you end up chasing small European suppliers or contacting university spinouts.
With a niche chemical, marketplace trust becomes critical. I’ve learned through the occasional misstep — getting a strangely labeled bottle or a questionable purity report — that chemistry carries more risk than a casual online purchase. If someone posts a bulk lot on an unfamiliar website, that doesn’t instill confidence. Google’s own E-E-A-T guidelines urge consideration of a vendor’s experience and trustworthiness, and that’s advice I stick by even for academic purchases.
I check if a company has regulatory verifications, published safety data, and customer service that will respond to requests for batch analyses. If a chemical firm can share a certificate of analysis, batch history, and proper hazard documentation – it’s a good signal. You can often spot a red flag with suppliers who skip these steps or avoid traceability questions.
Compounds like 3-Ethoxy-4,6-Difluoro-Dibenzothiophene don’t just float freely through the marketplace. Chemical regulation aims to prevent diversion into illegal or dangerous uses. Reputable vendors check credentials, require institutional addresses, and sometimes request project details. That can feel like a headache, but these checks line up with responsible science. In my own research days, paperwork slowed projects sometimes, though it also cut down on reckless handling.
As of 2024, most reliable suppliers restrict shipments to academic, pharmaceutical, or vetted industrial buyers, especially across country borders. Students or researchers hoping for “no questions asked” should prepare for disappointment or, worse, to fall into an unsafe gray market. The consequences — ranging from delayed shipments to outright scams — tend to outweigh any time saved.
For those consistently needing specialty compounds, direct communication with suppliers works better than automated web orders. Over years of lab projects, I found more success with phone calls where you can ask about synthesis routes, bulk discounts, or even batch customization. Industry directories like ChemSpider and MolPort sometimes help by listing alternative suppliers, though always use these as a first step rather than a final answer.
Collaboration helps, too. Some labs pool resources or share supplier contacts. Larger research institutes often leverage purchasing consortia for hard-to-source chemicals. If nothing else works, working with a contract research organization for custom synthesis can get a project back on track, albeit at a cost. In my view, sourcing obscure chemicals builds patience and negotiation skills faster than any textbook exercise.
Sourcing 3-Ethoxy-4,6-Difluoro-Dibenzothiophene reflects both the hurdles and the careful work behind chemical research. Trustworthy suppliers, strong documentation, and good communication stand out as the best way forward.
| Names | |
| Preferred IUPAC name | 3-ethoxy-4,6-difluorodibenzo[b,d]thiophene |
| Other names |
3-Ethoxy-4,6-difluoro-dibenzothiophene 3-Ethoxy-4,6-difluorodibenzothiophene |
| Pronunciation | /ˈθriː ɪˈθɒk.si ˌfɔːr sɪks daɪˈfluːəroʊ daɪˌbɛnzoʊˈθaɪ.oʊfiːn/ |
| Identifiers | |
| CAS Number | 1445847-05-7 |
| 3D model (JSmol) | `3d7c102zc00SeCcc(cc1F)cc(F)c1OCC` |
| Beilstein Reference | 1466120 |
| ChEBI | CHEBI:141485 |
| ChEMBL | CHEMBL3702081 |
| ChemSpider | 19879108 |
| DrugBank | DB08348 |
| ECHA InfoCard | ECHA InfoCard: 100.266.367 |
| EC Number | 1019272-24-8 |
| Gmelin Reference | 162642 |
| KEGG | C20649 |
| MeSH | D000072689 |
| PubChem CID | 16075012 |
| RTECS number | VL8234000 |
| UNII | V77K0J0IS0 |
| UN number | Not assigned |
| CompTox Dashboard (EPA) | DTXSID30612244 |
| Properties | |
| Chemical formula | C14H10F2OS |
| Molar mass | 290.30 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 1.44 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | 2.8 |
| Vapor pressure | 0.0000326 mmHg at 25°C |
| Acidity (pKa) | pKa = 6.86 |
| Basicity (pKb) | Basicity (pKb): 11.56 |
| Magnetic susceptibility (χ) | -72.73×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.612 |
| Dipole moment | 3.61 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 288.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | No ATC code |
| Hazards | |
| Main hazards | Harmful if swallowed, causes skin and eye irritation, may cause respiratory irritation |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P305+P351+P338, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-1-0-Health:1-Flammability:1-Instability:0-Special: |
| Flash point | 93 °C |
| NIOSH | NA |
| PEL (Permissible) | Not established |
| REL (Recommended) | 75 mg |
| Related compounds | |
| Related compounds |
3-Ethoxy-4,6-Difluorodibenzofuran 3-Ethoxy-4,6-Difluorodibenzoselenophene 3-Ethoxy-4,6-Dichlorodibenzothiophene 3-Ethoxy-4,6-Difluorodibenzothiophene-5-oxide 3-Methoxy-4,6-Difluorodibenzothiophene |