Long before 3-aminopyrrolidine gained recognition in synthetic chemistry circles, researchers poked through pyrrolidine’s structure searching for untapped utility. The parent molecule, first described in the mid-nineteenth century, made its mark by lending versatility to alkaloid synthesis and pharmaceutical design. Chemists paid more attention to modifying its nitrogen atom, stumbling across the aminated derivative during the 1970s. Scientists hoped to blend the backbone’s stability with increased reactivity, and early patents mirror that optimism. Density of experimentation picked up alongside rapid growth in medicinal chemistry and fine chemicals in the 1990s, using 3-aminopyrrolidine both as a building block and a subject of its own.
A bottle labeled 3-aminopyrrolidine contains a small organic block for many complex products. The core structure – pyrrolidine with an amino group on the third carbon – hints at a reactive spirit. Chemists blend it into synthesis paths chasing bioactive targets, polymers, and specialty materials. Bulk suppliers ship it in drums or bottles, usually as a clear to slightly yellow liquid that carries a discrete, ammoniacal scent. Most buyers are labs pushing for speed, flexibility, and purity above all. In my experience, a stockroom never leaves it lying around just anywhere; the need is precise, but demand spikes when new synthetic routes unfold.
3-Aminopyrrolidine sits at room temperature as a liquid, boils between 170 and 180°C, and dissolves freely in water, alcohol, or polar organic solvents. It sports a molecular weight just under 86 daltons. The compound, basic in nature, donates free electron pairs, making it eager for hydrogen bonding and rapid protonation. Handling it feels like working with a concentrated ammonia solution – gloves matter, as the skin tingles with even brief exposure. Beyond its basicity, the amine group opens doors for conjugation and functionalization, while the pyrrolidine ring stabilizes its shape and electron distribution. This isn’t just theory on a label, either: bench chemists notice it clings to glassware and often requires special care during transfer.
Manufacturers post purity on their product sheets, usually ranging from 98 up to 99.5 percent for research-grade material. Each shipment arrives with batch analysis details—water content, impurities like other amines, ash residue, and spectral signatures. Labels might state “3-aminopyrrolidine (CAS 504-29-0)” and warn of corrosivity along with storage below 30°C. Technicians check for clear labeling before issuing to anyone outside the synthetic chemistry crew. From what I’ve seen, the best labs insist on full traceability, as suspect purity means starting over and wasting precious time during crucial syntheses.
Making 3-aminopyrrolidine typically revolves around catalytic hydrogenation or reduction of its nitrile or nitro precursors. Many routes begin with 3-cyanopyrrolidine, subjecting it to high-pressure hydrogen in the presence of palladium or nickel catalysts. Others take the reduction path from 3-nitropyrrolidine, employing iron filings and hydrochloric acid or tin and ethanol solutions. Smaller-scale methods lean on sodium borohydride or lithium aluminum hydride reductions, but industrial nodes prefer reliability and scale. Lab veterans remember trial runs with excessive foaming or slow phase separation; a clean reaction still calls for careful workups, followed by fractional distillation to separate product from close-boiling impurities.
One reason chemists gravitate toward 3-aminopyrrolidine is its reactivity as a nucleophile. It couples easily with acid chlorides or anhydrides, lending itself to amide or urea formation—a route straight into drug candidates and agrochemicals. Reductive amination with aldehydes and ketones tweaks its profile, expanding the toolkit for medicinal chemists. Grignard reagents, once added slowly, yield secondary amines. The five-member ring structure stays tough during most transformations, but electrophilic substitution and N-alkylation experiments sometimes unlock unexpected reactivity. In my own bench work, the compound never feels boring—unplanned side reactions or ring opening events demand patience and solid analytical backup.
Industry catalogs and publications list 3-aminopyrrolidine under a handful of aliases: 3-AP, 3-Pyrrolidinamine, and 3-Aminotetrahydropyrrole. Chemical suppliers sometimes adopt systematized monikers like “1,2,3,4-tetrahydropyridin-3-amine.” International papers occasionally swap to German or French product names, but every bottle traceably links back to CAS 504-29-0. This is no trivial matter—mistaken identity in the chemical storeroom ends badly. Every experienced chemist learns to double-check, especially with pyrrolidine analogs around.
Gloves, goggles, and fume hoods are non-negotiable. Most safety sheets flag potential for burns, eye irritation, and respiratory trouble if inhaled as vapor. Good laboratory practice includes labeling transfer containers and keeping reactivity logs. Anyone who’s ever splashed a little on exposed skin doesn’t risk repeating it—intense tingling and redness remind you to respect the bottle. Chronic exposure worries toxicologists. Storage policies in the best labs call for tightly sealed bottles, frequent inspection for leaks, and restricted access. Regular refresher training keeps everyone sharp—complacency in handling amines costs days in clean-up or worse, a trip to the safety shower.
Drug discovery and development keep the demand for 3-aminopyrrolidine ticking. The amine backbone finds its way into antihypertensives, antivirals, and specialized oncology agents. Agrochemical companies reach for it, too, while chasing new seed treatments or pest-resistant coatings. Polymers and engineering materials sometimes turn to this compound for functional group incorporation in niche plastics. My colleagues talk about its role in constructing ligand libraries for screening—small-scale use can lead to millions of doses or hectares of crops after optimization. The opportunities aren’t limited to one sector, but the compound rarely spills into consumer goods due to its chirality and synthesis cost.
The compound pops up often in academic and industrial patent filings, tied to breakthrough synthetic methods or unique target molecules. Medicinal chemists love tweaking the amine location or ring size, probing for pharmacological surprises. In major pharmaceutical pipelines, combinatorial approaches draw on 3-aminopyrrolidine as a dependable node. Computational chemistry models also feed into lab work, hinting at new bioactivities or binding affinities through virtual screening. Sometimes, small pilot batches spin out in months as the route from hit to lead morphs. The feedback loop between chemistry, modeling, and biology turns the compound from a mere “reagent” into a stepping stone for breakthroughs. Those watching R&D closely know to expect papers linking the molecule to novel kinase inhibitors or immunomodulators in years ahead.
Animal studies flag moderate oral toxicity in rats and mice. Exposure above threshold levels brings respiratory irritation, headaches, and liver enzyme changes in chronic models. Inhalation—especially in unventilated spaces—can spiral into more severe problems. Some cell culture lines show cytotoxicity at micromolar concentrations, flagging caution for anyone hoping to scale up. Regulatory agencies push for cleanup protocols and medical monitoring in facilities using 3-aminopyrrolidine regularly. From personal experience, it pays to have spill kits and first aid within reach, and to brief every newcomer to the lab on its hazards, no matter how routine the synthesis schedule gets.
Nobody sees the spotlight fading anytime soon. Structural chemists forecast new derivatives of 3-aminopyrrolidine winding their way into targeted therapies and diagnostics. Biotech startups and scale-up chemists eye it when designing CRISPR delivery tools or bifunctional drug linkers. Regulations tighten slowly as new safety data emerges, but demand from evolving pharma needs tends to outpace restrictions. Some research teams lean on green chemistry, rolling out catalytic and solvent-free syntheses to lower risk and cost. I expect to see more automated handling systems slide into routine use where amines like 3-aminopyrrolidine matter most, blending safety, speed, and adaptability into future workflows.
3-Aminopyrrolidine might sound like a handful, but its formula keeps things pretty simple: C4H10N2. Its molecular weight checks in at 86.14 g/mol. For most chemists, this is basic info, yet these numbers shape countless choices behind the scenes.
Some people think of chemicals only as symbols and numbers, but in practice, every detail—like that formula or its precise molecular weight—becomes part of the foundation for planning reactions and weighing out materials. In the middle of a synthesis project, the difference between 86 and 86.14 g/mol can influence how accurately new compounds are made. Anything less than careful attention to formula and weight might start a cascade of mistakes—incorrect molar ratios, wasted reagents, or unpredictable results. I remember working late during my grad school days, hurriedly prepping solutions for an assay and skimping over decimals in weights. The results came out weird, and that error backtracked to ignoring a “tiny” 0.14 g/mol. Those small numbers do add up.
3-Aminopyrrolidine, with its two nitrogen atoms snug in a five-membered ring, draws interest because it serves as a building block for larger, more complex molecules. Research teams chasing new pharmaceuticals rely on foundational compounds like this one. It’s not just about curiosity—a simple molecule like C4H10N2 finds its way into reactions creating antiviral, anti-inflammatory, and neurologically active compounds. These transformations start and end with clean, reliable source materials.
Having the structure and weight on hand streamlines ordering and prepping stock solutions. You can picture a synthetic chemist double-checking the math before weighing out samples. Skipping those checks means risking failed batches. No one wants to toss hours of work because the original measurements were sloppy.
Chemical research always dances on the edge of precision. I’ve seen talented students frustrated just because they grabbed the formula for the wrong isomer or misread the weight. It turns into a domino effect. Delivering a new compound to the analytical core takes longer, and funding gets burned on repeat experiments. Mistakes also eat into the time set aside for validation steps, especially for projects with tight turnarounds.
This is not an issue unique to my own experience. Reports by the American Chemical Society have highlighted that about 20% of synthesis errors trace back to inaccurate stock calculations. It isn’t flashy work—no one wins a prize for reading a label right, but this kind of detail lets the breakthroughs happen.
Labs can cut down mistakes by keeping clear references. Use printed charts listing formulas and weights so everyone can double-check quickly. Sharing best practices about checking these numbers during group meetings saves headaches later.
Even straightforward info like the formula C4H10N2 and its molecular weight powers much of the daily work for chemists. It’s tempting to gloss over these details, but every step in a synthesis leans on them. Whether you’re a new student in the lab or a seasoned researcher chasing your next publication, accuracy at this level frees you up to think about the big picture, not scramble over basic math. That clarity helps keep research on track, gets new ideas out faster, and builds the trust other teams need when they use your data in the future.
Most folks probably haven't thought much about 3-Aminopyrrolidine unless they work in a lab, but this small molecule shows up more often than you'd expect, bridging chemistry and real-world uses. With its compact ring structure and flexible amine group, chemists like having it in their toolkit, especially in drug development and material science.
In my early days working closely with pharmaceutical researchers, I watched them reach for 3-Aminopyrrolidine nearly every time a project called for a nitrogen-rich backbone in potential therapy. The compound lives up to its reputation as a handy building block for designing molecules that target disease. One common routine involves attaching this fragment onto other structures to create new antiviral or anticancer drugs. Some advanced antipsychotic medications can trace their roots to molecules built using its framework.
Companies exploring new antibiotics rely on its versatility. The nitrogen in 3-Aminopyrrolidine gives chemists a chance to improve how drugs slip past bacterial defenses. It’s not all ‘flagship medicine stuff’—some smaller projects involve using it to make compounds that help control reactions in living systems, acting as inhibitors or blockers inside therapeutic drugs.
People in agriculture fight an endless battle against pests and weeds, and chemistry lends a serious helping hand. I remember reading about pesticide developers who used 3-Aminopyrrolidine as a key ingredient in making new formulas that stop plant diseases or insect infestations. Certain fungicides, designed to halt destructive blights before they take hold, use molecular fragments that start with this compound. By tweaking the core structure, scientists aim for molecules that break down quickly after doing their job, leaving less worry about environmental buildup.
It’s not just medicine and crops—3-Aminopyrrolidine also finds a place in making specialty plastics and coatings. If you’ve ever handled a device with a smooth, fingerprint-resistant surface, there’s a chance you’re touching materials made or modified with building blocks related to this compound. The amine group allows companies to attach other chemical features, giving materials extra strength or resistance to damage.
Handling 3-Aminopyrrolidine calls for care. It can irritate skin, so safe working conditions stay important. Another issue involves making the compound cleaner and greener. Chemical processes still lean on non-renewable sources, driving researchers to explore more sustainable production methods. Sometimes, I’ve talked with chemists who dream about starting from plant-based ingredients instead of relying on petroleum.
Having this piece in the chemist’s toolbox speeds up discovery in labs all over the world. From fighting diseases to protecting crops, 3-Aminopyrrolidine continues to show its worth. The next phase involves finding better, safer ways to make and use it—because every step toward greener chemistry feels like progress worth celebrating.
People who work in labs know that so much of their job relies on the quality of their chemicals. Every small test and big research project can fall apart if someone skips over the details. I remember early in my career, I grabbed a bottle of something with a label that looked official. The experiment failed, and then I took a closer look: the purity didn’t match up with the paperwork. Turns out, “close” just doesn’t cut it with lab work. 3-Aminopyrrolidine is another one of those chemicals—if your product is not up to spec, things don’t go as planned.
Most suppliers list 3-Aminopyrrolidine at around 98-99% purity. Sometimes, a small company on a budget might think that’s good enough. To a point, it is. For many reactions, a couple of percent in the wrong direction won’t sink the ship. The bigger issue comes up in pharma work, or when tracing impurities that could mess with biological data. Some research teams push hard for higher—99.5% or even more. That’s not just arbitrary. In the last five years, trace contaminants have caused enough problems that groups will gladly pay more for assurance.
It helps to know that “purity” isn’t just about what’s missing. It’s also about what’s there, even if it’s hiding. With 3-Aminopyrrolidine, common impurities are often leftover solvents or bits of pyrrolidine derivatives that didn’t get fully cleaned out after synthesis. These can lead to mystery peaks on an NMR or throw a whole process off down the line. None of this seems dramatic until an entire batch of an active pharmaceutical ingredient becomes worthless. Labs end up spending more on cleanup than they saved buying the cheaper starting material.
Low-purity chemicals can ruin reputations and budgets. Sometimes, that means a reaction that never runs right or waste that counts against your green chemistry goals. A few years back, a colleague of mine lost weeks trying to chase down the source of a single unexpected side product. In the end, it wasn’t the process; it was a contaminant in the batch of reagent. By then, they’d burned through time and funding.
Truth is, solutions don’t need to be complicated. Always ask for certificates of analysis and not just purity percentages—actual batch data makes a difference. Try to build relationships with suppliers who answer questions clearly, not just the ones with the lowest price tag. If something goes wrong, a responsive supplier can help you track down the source. I’ve found that investing in a little extra testing at arrival, using your own analytical methods, saves a lot of hassle. If your projects are sensitive, buy at the purity grade above what you think you need. It’s harder to fix contamination than to avoid it, and the big costs usually hide behind the mistakes that seem smallest at the start.
3-Aminopyrrolidine doesn’t stand out at first glance—just another chemical name in a lab supply catalogue. For folks working in labs or chemical supply, though, these details begin to matter fast. This colorless to yellowish liquid forms the backbone of several pharmaceutical and agrochemical syntheses. Touching it or breathing in the fumes isn’t just risky—it can turn a regular workday upside down with adverse health effects. Eye, skin, or lung irritation isn’t a far-off possibility; stories in chemical safety forums pop up from people caught off guard by a splash or a whiff.
Get careless with storage and trouble follows. 3-Aminopyrrolidine doesn't handle open air or heat well. It can absorb moisture, and oxygen only makes it degrade faster. From my time in the storeroom, I learned it's best to keep these sorts of amines in a cool, dry cabinet—far away from sunlight or heat sources. Temperature swings wreck the quality, and nobody wants a degraded sample wrecking their next reaction.
Another point centers on secure, sealed containers. Exposure to air makes containers sweat and drip, which can leach the chemical and boost the risk of contamination. I once found a bottle with a loose cap, leaking into the cardboard tray—mess and cleanup followed, and so did wasting what could have been a critical batch for a project. Stores need secondary containment for spills—a simple plastic tub works wonders for catching drips. Clearly labeled containers make sure nobody grabs the wrong bottle mid-rush.
Direct contact with 3-Aminopyrrolidine doesn’t end well. Spills on the skin lead to irritation, sometimes a rash. Vapors can sting the nose and throat, so it pays to gear up. Lab coats, gloves, and goggles don’t just look the part—they spare you the pain and medical headaches. From years of experience, I’ll say cotton gloves soak up spillage, which only makes the burn last. Nitrile works better, no questions asked.
Room ventilation matters. Running this chemical in a fume hood is a must. A classroom without proper airflow turns minor mistakes into big problems. The fume hood filters keep harmful vapors out of the breathing space, and persistent odors hint at buildup. One time our vent system failed, and a sharp, fishy smell lingered in the air… nobody got work done until maintenance fixed it and the room aired out.
Disposal brings its own headaches. Pouring chemicals down the sink seems easy, but it chokes up pipes and throws safety out the window. Waste labeled for amines—usually in a dedicated bottle—heads straight for professional treatment. Sloppy disposal brings EPA fines or, worse, injuries from leftover residue.
A spill kit in the corner, stocked with absorbent pads and neutralizers, makes cleanup less of a panic situation. Training everyone to handle spills, not just safety staff, keeps little messes from becoming major incidents. Emergency showers and eyewash stations nearby save precious seconds if splashes hit the wrong spot. Stories in the industry regularly reinforce that momentary carelessness costs dearly, both in time and health.
Getting casual about toxic chemicals leads to stories that nobody wants to retell. Mark your bottles, keep your storage space tidy, and walk through potential accident spots. Cross-check with the SDS every time you change batch sizes or try out a new application. Build up habits with consistent routines, and it’ll take far less effort to keep 3-Aminopyrrolidine in line—and staff safe.
I’ve met plenty of chemicals in the lab, but 3-Aminopyrrolidine keeps my respect. Built from a four-membered ring with an amine group, this compound carries promise for pharmaceuticals and organic synthesis. But promise comes with a price. Handling 3-Aminopyrrolidine without proper attention isn’t smart.
Contact with this chemical can irritate eyes, skin, and even bother your respiratory tract. People sometimes ignore tiny splashes during quick bench work, thinking soap and water fix everything. I know from experience, a simple fume from amine compounds can leave you coughing or your nose stinging for longer than expected. The mere mention of an amine functional group often means that a strong, sometimes fishy odor will hit before any other effects show up. Breathing it in, even in small amounts, begins to feel like you’ve walked through a cloud of household cleaner—but this cloud won’t let up right away.
Working around 3-Aminopyrrolidine without gloves, goggles, and solid ventilation means gambling with irritation. For those who dismiss these as minor consequences, it’s important to remember: repeated contact might lead to deeper skin issues, or allergic reactions if you’re unlucky. For people with asthma or allergies, even brief breathing of vapors can worsen their symptoms fast.
Swallowing it, which should not happen in any proper lab, ends up worse—nausea, vomiting, and central nervous system effects are no joke. Absorption through skin won’t necessarily send you to the hospital each time, but once is enough to convince most to double-check their PPE.
Let’s face it, chemical containers sometimes leak, especially with older stock. 3-Aminopyrrolidine should stay in tight, labeled bottles, away from acids and oxidizers. Acids in close contact could trigger nasty reactions or release dangerous gases.
Spill happens? Do not reach for paper towels like it’s a cup of coffee. You want proper gloves—think nitrile, not bare hands—and work area ventilated. In bigger spills, call for backup, use an absorbent meant for hazardous chemicals, and ditch clothes that get drenched.
Some folks argue that small-scale research doesn't justify big investments in safety infrastructure. Still, I’ve seen too many colleagues missing from the lab due to avoidable accidents. Fume hoods, routine training, and quick access to eyewashes and showers cut injury rates and don't slow anyone down once you’re used to using them.
MSDS (Material Safety Data Sheets) exist for a reason. Every time 3-Aminopyrrolidine leaves storage, bringing those sheets up offers a quick reminder of first aid advice and fire risks. Amine compounds can catch fire more easily than folks expect, and fires in a lab filled with other reactive stuff can spiral out of control.
There’s a push to replace hazardous chemicals with greener, less risky alternatives where possible. In plenty of cases though, compounds like 3-Aminopyrrolidine do a job that nothing else handles as well. Until safer choices become practical, following strong safety habits—not improvisation—sets the best example for everyone in the lab.
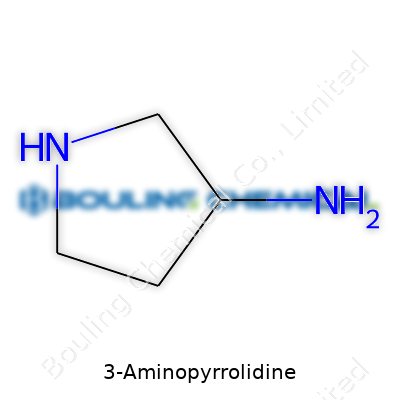
| Names | |
| Preferred IUPAC name | 3-Aminopyrrolidine |
| Other names |
3-Aminopyrrolidine Pyrrolidin-3-ylamine |
| Pronunciation | /ˈθriː-əˈmiːnəˌpɪˈrɒlɪˌdiːn/ |
| Identifiers | |
| CAS Number | 13035-19-3 |
| 3D model (JSmol) | `3D JSmol model string for 3-Aminopyrrolidine`: `3D;AQIDBAUGBwgJCgsMDQ4PEBESExQVFhcYGRobHB0eHyA=` |
| Beilstein Reference | 2350887 |
| ChEBI | CHEBI:85166 |
| ChEMBL | CHEMBL138246 |
| ChemSpider | 68275 |
| DrugBank | DB04126 |
| ECHA InfoCard | 100.153.864 |
| EC Number | 220-083-9 |
| Gmelin Reference | 67767 |
| KEGG | C14379 |
| MeSH | D042742 |
| PubChem CID | 122313 |
| RTECS number | SE8610000 |
| UNII | KSZ1J9629A |
| UN number | UN2734 |
| Properties | |
| Chemical formula | C4H10N2 |
| Molar mass | 87.14 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Amine-like |
| Density | 1.06 g/mL at 25 °C(lit.) |
| Solubility in water | Soluble |
| log P | -0.54 |
| Vapor pressure | 0.38 mmHg (25°C) |
| Acidity (pKa) | 10.10 |
| Basicity (pKb) | 2.97 |
| Magnetic susceptibility (χ) | -54.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.518 |
| Dipole moment | 1.57 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 253.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -44.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3768.8 kJ/mol |
| Pharmacology | |
| ATC code | Not assigned |
| Hazards | |
| Main hazards | Harmful if swallowed, causes severe skin burns and eye damage, harmful if inhaled. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | **["GHS07"]** |
| Signal word | Warning |
| Hazard statements | H302, H314 |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | 2-3-1 |
| Flash point | 100°C |
| Lethal dose or concentration | LD50 (Oral, Rat) 970 mg/kg |
| LD50 (median dose) | LD50 (median dose) for 3-Aminopyrrolidine: Oral rat LD50 = 239 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 1000 mg/L |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
1-Aminopyrrolidine 2-Aminopyrrolidine 4-Aminopyrrolidine Pyrrolidine 3-Aminopyrrolidine dihydrochloride N-Methylpyrrolidine |