The story of 3-Aminopyrazine-2-carboxylic acid stretches back to the mid-20th century, built around a growing understanding of pyrazine derivatives. Researchers in organic chemistry took interest due to the versatility of pyrazine rings and their clear impact on medicinal chemistry. Early syntheses borrowed from classical methods, often producing small yields, which frustrated those working in both academic and industrial laboratories. Over time, optimization followed, driven by the promise of new antibacterial, antifungal, and agricultural products. Generations of chemists passed down modifications, accumulating a body of knowledge that now supports its routine preparation. Looking back, the journey saw basic research in heterocyclic chemistry shift toward targeted synthesis, analytical advances, and growing industrial uptake.
The compound 3-Aminopyrazine-2-carboxylic acid has earned a place on the shelf of any serious lab working with fine chemicals, agrochemical intermediates, or potential pharmaceuticals. Its unique pyrazine backbone, with strategically placed amino and carboxylic acid groups, makes it attractive for both direct application and further modification. Scientists value its reliability as a building block and intermediate, particularly when constructing molecules where electron distribution and hydrogen bonding are key. The molecule doesn’t just serve a single market; it brings practical value to drug discovery, diagnostics, and even niche materials research.
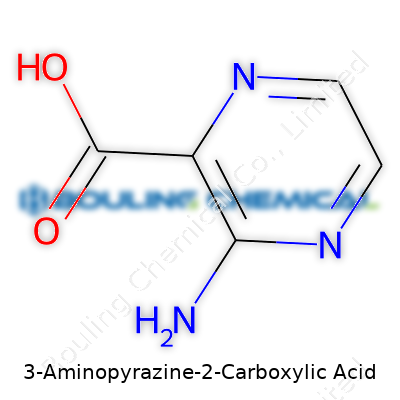
This solid appears off-white or pale yellow and sports a molecular formula of C5H5N3O2, with a molar mass around 139.12 g/mol. Its melting point sits between 260°C and 270°C, which is high enough to keep worries about accidental melting to a minimum during handling or reactions. The chemical structure features a six-membered aromatic ring, boasting one amino group at position three and a carboxylic acid at position two. Solubility in water stays moderate, though it swells in DMF or DMSO, and those working with the solid must watch for its tendency to form strong intra- and intermolecular hydrogen bonds. Acidity corresponds to the carboxylic group, while the ring also supports nucleophilic substitutions due to the electron-withdrawing nature of the adjacent nitrogen atoms.
Quality-conscious users often call for purity above 97%, with HPLC and NMR serving as mainstays in confirming batch consistency. Labels on commercial products cite the CAS Number 7415-67-6, storage instructions (cool, dry places), and warning information tied to its irritant risk profile. Package sizes stretch from grams for academic work up to kilograms for process research teams. Certificates of analysis detail trace impurities, water content by Karl Fischer testing, and the permissible heavy metal concentration—key variables for regulatory compliance. Reference to batch numbers and synthesis conditions make traceability on par with best practices for research and safe industrial deployment.
Synthesizing 3-aminopyrazine-2-carboxylic acid usually begins with a substituted pyrazine precursor, often pyrazine-2-carboxylic acid or an analogous ester. Amination of the pyrazine ring can take several approaches, but direct introduction of the amino group at the three position remains dominant, using reagents like ammonia or amines in polar solvents under elevated temperatures. Reduction of nitro groups or other protected intermediates extends the synthetic options. Process chemists find that optimizing pH, solvent, and reaction times brings higher yields and cleaner products, reducing the need for laborious purification steps. Industrial players push toward greener, catalytic routes, not just for efficiency but to cut down on waste and energy consumption.
The molecule stands out for its chemical flexibility. Carboxyl groups open opportunities for amide coupling and esterification, fundamental in producing peptide mimetics and drug candidates. Amino groups enter acylation, sulfonation, and diazotization, connecting to dyes, agrochemicals, and advanced materials. Selective substitutions on the pyrazine ring allow design of novel analogs, with medicinal chemists using every handle the molecule offers. One vivid example lies in linking the carboxylic acid to form prodrugs, altering pharmacokinetic profiles to boost bioavailability. Academics likewise use the compound’s core for preparing coordination complexes, exploring catalysis or materials science possibilities.
In shipping orders and scientific papers alike, 3-aminopyrazine-2-carboxylic acid may appear as “3-APCA,” “2-pyrazinecarboxylic acid, 3-amino-,” or by catalog numbers from major suppliers. Some texts reference it as “3-aminopyrazine-2-carboxylate acid” or “pyrazinecarboxylic acid, 3-amino,” reflecting slight shifts in naming conventions over time or by region. These synonyms shape literature searches, enforce consistency across supply chains, and guide regulatory submissions, helping evade confusion amid similar-sounding analogs.
Every lab user quickly learns to respect this material, not for high acute toxicity but because safety protocols help avoid skin, eye, and respiratory irritation. Lab coats, gloves, and properly vented hoods strike a balance between productivity and well-being. Spills or contact with incompatible reagents risk forming mild irritants—quick cleanup and timely disposal become habits. Packaging follows hazardous material guidelines, with labeling echoing globally harmonized system standards. Training on storage stability and container integrity guards against moisture ingress, which might degrade the material or complicate downstream use. Industrial settings introduce more formal precautions, involving exposure monitoring, material safety data sheets, and routine risk assessments before scaling up processes. Shipping remains subject to standard transport regulations, particularly for bulk quantities destined for pharmaceutical or specialty chemical applications.
Opinions on its main value depend on the professional background. Pharmaceutical researchers see it primarily as a precursor for drugs targeting tuberculosis, other bacterial infections, or rare metabolic conditions. Agrochemical companies use it for herbicide and fungicide development, leaning on its compatibility as a core structure for synthetic analogs. Diagnostics and analytical chemistry benefit from derivatives with strong affinity for metal ions, supporting imaging or quantification. Recent years saw a surge in investigating its behavior in polymer chemistry, giving rise to new smart materials that respond to pH or other triggers. The reach of its applications reflects a broad commitment to extracting practical outcomes from chemical innovation, building value not just for industry but for public health and research communities.
Ongoing research finds companies and universities fine-tuning both synthesis and application. Many current efforts look to shorten synthetic routes or use renewable feedstocks. At the same time, teams continue expanding structure-activity relationships, combing through analog libraries for compounds with greater antimicrobial or antifungal properties. Analytical chemists leverage NMR, MS, and crystallography to better understand tautomerism, hydrogen bonding, and solid-state forms, each affecting how the compound performs in real-world settings. Collaboration between industry and academia helps move promising analogs from the bench to preclinical trials, pooling resources in a mutually beneficial cycle of discovery. Companies also invest in education and training, building up the next wave of scientists fluent in both theory and practice surrounding this and related molecules.
Studies on this compound show relatively low acute toxicity, yet chronic exposure concerns persist, especially for workers handling large volumes or in settings where controls may slip. Toxicologists track metabolic breakdown in animal models, noting any formation of genotoxic metabolites. Environmental safety teams evaluate persistence in soil and water, seeking to minimize the load of synthetic intermediates in ecosystems. Many journals require rigorous safety data before publishing new applications, reflecting growing social and regulatory pressure to close knowledge gaps on risk factors. For users, this means monitoring for updates in allowable exposure levels, keeping informed of new data as regulatory landscapes change.
Expanding markets for personalized medicine likely boost demand for building blocks like 3-aminopyrazine-2-carboxylic acid, encouraging new investment in next-generation synthesis and analytics. The search for antimicrobial solutions in the face of drug resistance continues to drive structural modification, with fragment-based drug design making creative use of this scaffold. Green chemistry is shifting the production model, with catalysis, solvent recycling, and renewable inputs quickly moving into the mainstream. Meanwhile, educational and open data initiatives give a wider audience a chance to access best practices and contribute fresh ideas. The journey of this molecule, from bench curiosity to core industrial intermediate, says a lot about what's possible when basic science, practical need, and shared knowledge push each other forward.
3-Aminopyrazine-2-carboxylic acid might sound like a mouthful, but in the lab, it plays a small, crucial role. This compound, often found on the bench of pharmaceutical chemists, acts as a raw ingredient for synthesizing more complex molecules. I’ve seen it turn up on shopping lists for researchers aiming to build experimental antibiotics or anti-cancer drugs.
Research in medicinal chemistry leans heavily on small structures with nitrogen atoms because they often bind well to biological targets. 3-Aminopyrazine-2-carboxylic acid brings these features together. Its structure gives chemists flexibility for chemical reactions, letting them attach different side groups or link it into larger ring systems. Walk through any chemical catalog, and you’ll notice how often companies offer this compound for just that purpose—building new drug candidates from the ground up.
New medicines start with compounds like these. The pyrazine core shows up in drugs for everything from tuberculosis to various cancers. I’ve read studies where scientists synthesize dozens, sometimes hundreds, of derivatives before finding something that seems promising in the lab. Without reliable, affordable sources of these “starting materials,” that work gets a lot harder.
Beyond medicine, specialty dyes and agricultural chemicals benefit from its structure. It’s not always the main attraction, but its chemical backbone provides stability and tuning options for color or biological activity. In my own undergraduate experience, we used a similar scaffold to fine-tune the properties of a fluorescent dye, and the difference a simple carboxylic acid functional group makes can be huge.
Safety always comes into play when dealing with compounds like this. Even more so when the final product heads into animals or humans. Labs run exhaustive tests, measuring impurities down to a sliver of a percent. Consistent quality across batches matters because a single contaminant can ruin months of work or skew results. In that sense, the reputation of a supplier often decides which brand gets stocked in the storeroom. In interviews, experienced researchers have shared stories of product recalls or test failures traced back to minor flaws in a starting reagent.
On top of that, sourcing these fine chemicals isn’t always simple. Trade issues or disruptions in manufacturing can delay projects for weeks or months. Some companies keep backup suppliers for common intermediates like this just to keep projects moving. The world noticed these vulnerabilities during recent global supply chain crunches. Drug discovery isn’t just about smart people in white coats—the whole ecosystem behind those experiments matters.
Fixing these bottlenecks could speed up innovation in both academia and industry. Investment into domestic chemical manufacturing provides a security net. I’ve met professors who call for more government-backed stockpiles of high-demand research chemicals, especially in countries with few production plants. Large-scale recycling programs for unused chemicals might also help lower costs and reduce hazardous waste, something the field struggles with.
3-Aminopyrazine-2-carboxylic acid won’t ring bells outside a research lab, but its influence ripples out into real-world treatments and products. The behind-the-scenes work with these raw materials often determines how quickly, safely, and affordably new discoveries reach the people who need them most.
Walking through any modern research lab, I notice how chemistry shapes medicine, materials, and technology. Scientists track down tiny pieces of chemicals, searching for promising building blocks. One molecule I’ve seen discussed repeatedly in research circles is 3-Aminopyrazine-2-Carboxylic Acid. Peeling back its layered name, this compound reveals a story that matters far beyond the academic pages.
By specifying C5H5N3O2 as the molecular formula, chemists sum up the essence of this molecule: five carbons, five hydrogens, three nitrogens, and two oxygens bound together in a unique shape. In my own work, finding a structural formula like this means anyone in the world—chemist or not—can begin to picture the skeleton of the compound. It helps researchers, pharmacists, and regulatory bodies stay on the same page, cutting down on confusion and errors. Years ago, I watched two labs waste months cross-checking duplicate chemicals simply because one group jumbled the naming system. Precise formulas dodge those headaches.
The formula gives more than trivia—it opens the door for practical applications. Pyrazine derivatives often show up in medicines and agricultural agents. The amino group and carboxylic acid attached to that pyrazine ring mean this compound can take part in hydrogen bonding, metal coordination, and chemical synthesis. In pharmaceutical development, these features act as a Swiss Army knife. A clear formula like C5H5N3O2 lets computational chemists run simulations, hunting for activity against enzymes or pathogens without having to guess its properties.
Looking back at projects where molecular identification stalled progress, missed formulas slowed innovation. Mistakes with a single atom can halt a trial, poison an experiment, or, in grim cases, risk patient safety. Getting the exact count and type of atoms—like knowing just how three nitrogens fit with five carbons and the rest—makes everything downstream more reliable.
Chemical names get tangled across borders and industries. Everyone from global regulatory agencies to local professors wants to avoid missteps. Standardized formulas, especially those confirmed in international databases and peer-reviewed articles, take guesswork out of cross-checking efforts. I’ve seen students convince themselves they had a breakthrough compound, only to realize translation errors forced them to start over. Sharing the correct molecular formula keeps collaboration honest and productive.
Handwaves and vague descriptions won’t cut it. C5H5N3O2 pinpoints what 3-Aminopyrazine-2-Carboxylic Acid really is. For anyone involved in research, drug safety, or chemistry education, sticking with the facts allows progress. If we want to keep trust and safety front and center in chemical development, leaning on clear, verified molecular formulas remains one of the straightest paths forward.
Some chemicals demand particular respect, especially in research and industry labs. 3-Aminopyrazine-2-Carboxylic Acid isn’t flashy, but careful storage matters just as much for this compound as it does for more reactive substances. Anyone who’s worked with aromatic carboxylic acids knows—environment shapes stability and safety as much as purity or identity.
Moisture creeps in everywhere. Even a “sealed” jar can pull humidity from the air if it sits out, which leads to clumping, hydrolysis, or slow degradation. I’ve seen small bottles turn chunky after a few careless months. So, a lesson learned the hard way: keep the material tightly capped and use desiccators for longer-term storage. A classic silica gel or anhydrous calcium chloride pack offers peace of mind, especially in shared lab spaces where airflow and traffic can push open containers.
Temperatures matter too. Cool, stable environments cut down on chemical breakdown. Standard procedure calls for room temperature, but it pays to check the supplier’s safety data sheet. If it says “store in a cool, dry place,” that means a climate-controlled cabinet, not a sunlit countertop beside heat-generating equipment. Rapid changes from warm to cool speed up condensation, which means more moisture finds its way in.
While not all carboxylic acids degrade from light exposure, some aromatic compounds quietly see changes in color or purity over months under bright bulbs. Lab experience taught me to default to amber glass if sunlight or harsh overhead LEDs are around. Even if 3-Aminopyrazine-2-Carboxylic Acid doesn’t yellow right away, years of research show that photo-degradation is sneaky and slow. No point gambling with shelf life during long projects.
Everything comes down to clear labeling—date opened, initials, hazard symbols. I keep a habit of marking every container, after finding out the hard way what a mystery bottle does to productivity. Mixing storage with high-risk organics or incompatible acids sets the stage for confusion or, worse, cross-reaction. Flammables, oxidizers, and acids shouldn’t sit together. Separate shelving or even a labeled tote works. Most storerooms have guidelines for segregation, and they are written in response to more than a few accidents worldwide.
Safety isn’t just about PPE or emergency showers. Proper storage supports both lab staff and research outcomes. Compromised chemicals produce unreliable results, which can shape years of wasted work. Handling 3-Aminopyrazine-2-Carboxylic Acid thoughtfully recognizes that clean science runs in parallel with safe habits.
For anyone new in the lab or managing inventory at home, start with dry, cool, labeled, and segregated. Check caps, watch humidity, avoid sun, and track dates. Chemical safety begins on the storage shelf. That’s a lesson worth passing on—no exceptions.
3-Aminopyrazine-2-carboxylic acid pops up mostly in research labs and chemical manufacturing sites. It’s no household name, but for people working with organic synthesis or developing drugs, this compound means something. When I started in a chemistry lab, every new chemical sent me digging through safety data sheets, partly out of worry, partly curiosity. Safety always ranked above convenience; nobody wants to mess around and end up in the ER because they underestimated something small and white like this.
Looking at the chemical itself, most database listings, including PubChem and ChemSpider, show limited toxicity data. 3-Aminopyrazine-2-carboxylic acid hasn’t received as much scrutiny as common solvents or acids. There’s a story behind that—chemicals with massive commercial use tend to get more research thrown at them. Less use, less data. Some may see this as a sign it must be safe, which is a risky assumption. Just because something hasn’t hurt a lot of people doesn’t mean it won’t. Lack of data means we keep our guard up.
What we can piece together: the compound doesn’t show classic “hot zone” features like explosive volatility or acutely toxic fumes. Nothing suggests it’s corrosive to skin or eyes to the level of strong acids or bases. But we do know that many pyrazine derivatives can trigger irritations, especially in the lungs or skin if you inhale fine dust or spill large amounts. Extended lab exposure without protection invites unnecessary risk—itchiness, rashes, sometimes temporary breathing discomfort.
Basic chemical hygiene never goes out of style. A decent lab coat, gloves that can handle organics, and safety goggles: this routine has saved me and my peers from countless mishaps. Sometimes people skimp and work “just for a second” without gloves or masks; the odds catch up, usually at the worst moment. Spills, forgetfulness, and the chilling surprise when something stains your hand make for rough lessons. Consistency prevents small problems from turning big.
I always store these compounds in tightly sealed containers, away from food, acids, and oxidizers. Keeping chemicals in their own space, not lumped together on crowded shelves, stays non-negotiable. Most research guidelines flag “treat as potentially harmful unless proven otherwise.” This sort of caution kept my team’s accident records clean, and it sets the tone for younger chemists.
Systematic toxicity testing would settle the question for everyone. People working in regulatory agencies or industrial safety departments could push for more studies. Until then, treating unknowns with respect and using every safety tool at hand is not just smart, it’s a duty. Where substitutes exist that come with clearer risk profiles, using those makes sense—especially where regular exposure could add up over months or years. Open lab logs, honest incident reports, and regular safety drills sharpen everyone’s instincts.
Curiosity about every chemical I touch never faded, not after years in the lab. The drive for knowledge is personal, but so is the responsibility to prevent harm. Caution, information-sharing, and proper protection gear form the best defense against both old hazards and new unknowns.
Researchers dig deep into the potential of 3-Aminopyrazine-2-Carboxylic Acid, especially in drug discovery labs. This molecule carries features that fit right in with pharmaceutical development. Chemists often start with this compound when designing antiviral or anticancer candidates, because its core structure interacts well with many biological targets. In my experience, working with experimental cancer therapies, the pyrazine ring in this molecule proved valuable for modifying activity against specific cellular enzymes. Scientists find this backbone gives flexibility, letting them add side groups that change potency or reduce unwanted effects in test compounds.
Materials scientists constantly look for reliable pieces to build new molecules. 3-Aminopyrazine-2-Carboxylic Acid serves as a convenient building block for creating complex heterocycles, which are the foundation for several organic electronic materials and specialty dyes. Research teams use it as a starting piece for constructing luminescent materials, often seen in OLED displays or laser technologies. The ability to fine-tune electronic properties by tweaking this acid’s structure has opened doors in photonics research, an area growing rapidly as industries need smarter sensors and displays.
Farmers and agronomists face ever-changing challenges from pests and plant diseases, so new agrochemicals remain a priority. Folks working in agricultural research appreciate how 3-Aminopyrazine-2-Carboxylic Acid helps shape pesticide and herbicide development. The molecule’s skeleton slots into active agents that fight disease or harmful insects. In practical work, I’ve seen teams use it to create selective agents that target weeds without hurting crop yields. The logic comes down to tuning small molecular details to make compounds more precise in their action, saving money and lowering environmental impact.
Labs that explore the inner workings of cells or environment need sharp detection tools. 3-Aminopyrazine-2-Carboxylic Acid acts as a parent structure for molecular probes—those chemical “keys” that light up or bond only when a certain target is present. Chemists make sensors based on this acid to measure trace levels of metal ions or to monitor shifts in pH. These sensors find use in environmental monitoring, medical diagnostics, and industrial quality control. The acid’s chemical traits allow for easy modification, letting researchers adapt sensors for different jobs.
Handling any specialty chemical brings challenges. Concerns like workplace safety, purity, and cost shape how scientists and manufacturers approach compounds like this one. Commercial suppliers must maintain strong quality controls and clear documentation. I’ve seen labs fall short when ignoring minor impurities, which end up skewing results. People need to keep safety training updated and invest in better handling protocols. Collaborations among academic and industrial partners could lower costs and speed up access to innovative applications.
The story of 3-Aminopyrazine-2-Carboxylic Acid shows how one small molecule plays a big part across research and industry. Having worked at the border of chemistry and product design, I’ve noticed that focusing on fundamental building blocks speeds up problem solving, sparks new inventions, and leads to smarter, safer products. By pushing for cleaner, more transparent supply chains, the science community can keep improving results, sharing findings, and making sure new ideas lead to something valuable.
| Names | |
| Preferred IUPAC name | 3-aminopyrazine-2-carboxylic acid |
| Other names |
3-Amino-2-pyrazinecarboxylic acid 3-Aminopyrazine-2-carboxylate 3-Aminopyrazinecarboxylic acid |
| Pronunciation | /ˈθriː-əˌmiːnoʊ-paɪˈreɪziːn-tuː-kɑːrˈbɒksɪlɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | [64241-58-1] |
| Beilstein Reference | 120889 |
| ChEBI | CHEBI:103009 |
| ChEMBL | CHEMBL256043 |
| ChemSpider | 129849 |
| DrugBank | DB07706 |
| ECHA InfoCard | 03c95798-7cf6-4b0a-9475-94903a100ef8 |
| EC Number | NA |
| Gmelin Reference | 822277 |
| KEGG | C18504 |
| MeSH | D000077678 |
| PubChem CID | 14620506 |
| RTECS number | UD5950000 |
| UNII | 36CZG60Z2A |
| UN number | Not regulated |
| CompTox Dashboard (EPA) | DTXSID4076933 |
| Properties | |
| Chemical formula | C5H5N3O2 |
| Molar mass | 138.11 g/mol |
| Appearance | White to Off-White Solid |
| Odor | Odorless |
| Density | 1.59 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -1.5 |
| Vapor pressure | 6.3E-8 mmHg at 25°C |
| Acidity (pKa) | 2.1 |
| Basicity (pKb) | pKb = 11.62 |
| Magnetic susceptibility (χ) | -43.1 × 10⁻⁶ cm³/mol |
| Dipole moment | 3.55 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 148.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -180.7 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1399 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 272.2 °C |
| NIOSH | NA0400000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 1 mg/m³ |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Pyrazine 2,3-Diaminopyrazine Pyrazine-2-carboxylic acid 3-Aminopyridine 2-Aminopyrazine 3,6-Diaminopyrazine |