The story of 3-Aminohomopiperidine traces back to early explorations in heterocyclic chemistry when scientists pushed boundaries to find new ring systems with biological promise. In the 1960s, chemists looked beyond piperidine, hoping for fresh building blocks for pharmaceuticals and specialty materials. By the late twentieth century, thanks to advances like selective hydrogenation and improved protective group strategies, researchers managed to reliably synthesize homopiperidine derivatives and open a new chapter in nitrogen-containing heterocycles. Demand for custom starting points in drug discovery and material science fueled progress, with Eastern European and Japanese research groups often leading the charge to develop novel multi-step protocols.
3-Aminohomopiperidine stands out as a nitrogen-rich, saturated seven-membered ring decorated with a primary amine group. Chemists favor this scaffold for its versatility. As a starting material, it opens doors to new heterocycles, advanced polymers, and bioactive molecules. Many suppliers keep it in standard inventory for research use, sometimes offering custom batches for pilot plant work. Its value grows in research fields focused on central nervous system modulators, because the amine group becomes a handy point for developing analogs of psychoactive or analgesic compounds.
Under standard lab conditions, 3-Aminohomopiperidine typically appears as a white to pale yellow crystalline solid, with a faint amine smell reminiscent of fresh-cut grass. It dissolves in water and common polar organic solvents such as ethanol, and forms stable hydrochloride or sulfate salts for ease of handling during synthesis. The molecular formula, C6H14N2, puts its molecular weight just above that of common piperidine derivatives. Under basic conditions, the amine group reacts rapidly with electrophiles, while acidic environments may cause slow hydrolysis over days, which researchers need to monitor in long-term experiments.
Laboratory catalogs usually list 3-Aminohomopiperidine with purity above 97%, verified by HPLC and NMR. Labels include CAS number 63940-38-5, batch number, and supplier details. Material Safety Data Sheets detail storage at ambient temperature, with a cap on moisture ingress to prevent degradation. Analytical standards often accompany lots destined for regulated or pharmaceutical environments, including chromatograms and sometimes mass spectrometry fingerprints. Responsible manufacturers ship the material in sealed amber glass bottles, discouraging light-induced decomposition, and recommend secondary containment during storage.
The most common prep route begins with cyclohexanone, which undergoes amination through reductive amination with ammonia derivatives. Hydrogenation over Raney nickel converts unsaturated intermediates to the homopiperidine ring. Latest protocols exploit protecting groups to temporarily mask amine functionalities, making later steps more selective. Large-batch producers scale up reactions using flow chemistry, which allows for tighter control over temperature and residence time compared to batch processing. Sometimes biocatalytic steps join the synthetic sequence to improve atom economy and reduce hazardous waste, especially in green chemistry-oriented facilities.
The free amine group serves as a launchpad for modifications. It reacts swiftly with acid chlorides to give amides, and with isocyanates for urea derivatives. Many medicinal chemists use 3-Aminohomopiperidine as a scaffold for peptidomimetic drugs, introducing bulky substituents at the amine using alkylation. Its ring nitrogen, when oxidized, offers nifty routes toward N-oxides, leading to molecules with new pharmacological profiles. In polymer science, crosslinking of the amine enables creation of tough, high-performance materials suitable for coatings and membranes.
Anyone hunting for this compound online or in databases will notice a grab bag of synonyms. Common ones include 3-Azahomopiperidine, 3-Aminoazepane, and even 3-Aminohexamethyleneimine. Pharmaceutical catalogs sometimes list it under truncated versions or project codes, especially in internal research. Recognizing these names helps avoid confusion in procurement or when reading literature across different chemistry subfields.
Standard lab gloves and goggles remain the primary line of defense against exposure, because amines often irritate skin or eyes. Fume hoods ensure that vapors and dust stay far from airways, since even low-molecular-weight amines sometimes cause headaches or dizziness. Chemical waste protocols demand that unused 3-Aminohomopiperidine be neutralized and transferred to designated disposal, avoiding sinks and municipal drains. Some jurisdictions treat this compound within the same hazard class as other low-toxicity aliphatic amines, meaning shipping and workplace signage must follow strict rules. Spill response involves absorbent pads and prompt ventilation, rarely requiring escalation unless large quantities hit the bench.
Medicinal chemistry firms probe the molecule for antidepressant, anticonvulsant, and antipsychotic lead discovery. Its seven-membered backbone pops up in the early scaffolds of CNS modulators, sometimes outpacing six-membered analogs for BBB penetration. Outside the drug world, specialty chemicals makers have found use for it as a monomeric unit, introducing it in polymer backbones for more ductile, resistant plastics. Biomedical device manufacturers test its salt forms for antimicrobial coatings, harnessing the innate reactivity of the amine functionality. Even agrochemical R&D teams explore its ability to fit into new-generation insecticidal agents by combining with aryl sulfonyl chlorides or novel carbamates.
Bigger pharma names and deep-pocketed academic groups lead the search for structural analogs with higher potency and selectivity. Their publications show 3-Aminohomopiperidine turning up in screening libraries for both enzymatic and receptor targets. As high-throughput chemistry and AI-driven molecular design gain ground, demand for non-standard ring systems keeps rising. Some research pivots toward sustainable production, swapping precious metal catalysts for renewable biocatalysts. In advanced materials, physicists and engineers now study how amine-capped homopiperidines interact with graphene and other conductive surfaces, hoping to bake smart chemical triggers into next-generation electronics.
Animal models suggest moderate acute toxicity, higher than its six-membered cousins due to greater uptake and slower clearance. Samples rarely pass the blood-brain barrier efficiently, limiting risk but raising interest for those seeking targeted CNS action. Long-term exposure in rodents doesn’t show strong carcinogenic signatures, but subtle liver enzyme changes crop up, cautioning against casual use. Environmental impact scientists study its photodegradation and find breakdown products tend to mineralize quickly, so lab spills don’t collect in soil or groundwater. Regulatory agencies have yet to trigger stringent controls, largely because industrial use remains limited.
Researchers eye 3-Aminohomopiperidine as a launchpad for breakthroughs in neurotherapeutics, where unconventional scaffolds matter more than ever for IP protection and therapeutic advances. Pushes in sustainable chemistry may expand its supply chain, unlocking greener syntheses and ready adoption by industry. Software-guided molecular design, now a staple in leading labs, will lean on scaffolds exactly like this, offering fresh chemical space for patent cliffs dogging big pharma. As interdisciplinary teams team up—polymer scientists with med chemists, device designers with synthetic chemists—this molecule could cut across specialties and power fresh applications in markets still beyond today's horizon.
A lot of folks who spend their days working with molecules have probably heard of piperidine rings and all the tweaks chemists make to them. Tossing an amino group onto a chain isn’t just a trick in the lab—it can totally shift how a compound performs out in the real world, in everything from experiment tubes to the pill bottles folks use every morning. 3-Aminohomopiperidine doesn’t pop up in dinner conversations, but digging into its chemical structure gives you a front-row seat to why small changes make a big difference.
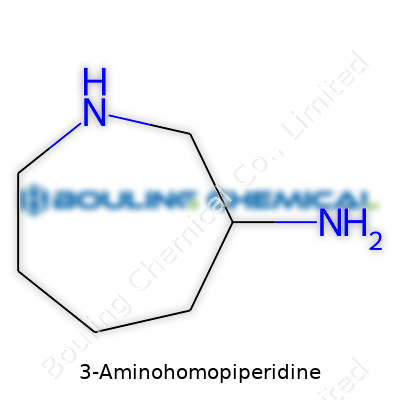
Take the backbone of this molecule. If you sketch it out, it isn’t just a straight line or a jumble of random atoms; it’s built around a seven-membered ring. That’s a step up from the six-membered piperidine ring so many know, giving it a little extra elbow room. The "homo" in homopiperidine signals the extra carbon hanging out in the ring, stretching it out and shifting the way the rest of the groups get attached. It’s not a tiny nitpick. Rings like this change the shape and the vibe, affecting flexibility and stability.
Stick an amino group (–NH2) on the third carbon—counting from the nitrogen that’s already in the ring—and you get the exact structure called 3-Aminohomopiperidine. Chemically, some folks would jot its formula as C6H14N2. You could draw it out as a heptane ring, swap out a carbon for a nitrogen atom, and fix another nitrogen to carbon-3 via the amino group. This isn't just for the sake of naming traditions. The extra –NH2 hanging off the side changes how this compound links up with others, adding a basic “sticky” point for reactions, whether it’s forming bonds or breaking them.
I’ve worked with different amines and ring systems, and it gets clear pretty fast that changing a ring size or moving an amino group changes what you can do with the whole molecule. Give a chemist 3-Aminohomopiperidine, and it suddenly unlocks a new toolkit. The extra ring size makes the molecule a little more wiggly, meaning it sometimes fits better into the targets found in living systems, whether that's for a new kind of medicine or a material science project.
Drug researchers look at a structure like this and immediately imagine ways to attach side groups or build up complexity. The two nitrogen atoms give it double trouble: one’s embedded in the ring, making it part of the core structure, while the other is ready to snap onto more complex fragments, helping researchers tune up how the compound acts inside a body. Bigger rings compared to classic piperidines often lead to changes in how the molecule slips through membranes or binds with proteins, which can help dial down unwanted effects.
One headache I’ve seen with seven-membered rings, like in homopiperidines, comes from trying to make them efficiently. Six-membered rings seem to practically assemble themselves, but when you want that seventh carbon, sometimes yields drop or you get unwanted byproducts. Every tweak to the process can make a big difference, from the temperature to the solvents and even the time you let things stir. Optimizing this step has tripped up more than one synthetic chemist over the years.
Teams working in industry sometimes get creative: using ring-closing reactions, dialing in protective groups, or changing the starting materials out completely. Bringing in modern tools—like automated purification, high-throughput screening, and computer-aided design—helps dodge a lot of the classic stumbles. As research continues, more cost-effective ways to create 3-Aminohomopiperidine keep emerging, and each improvement opens up new options in science and technology.
3-Aminohomopiperidine doesn’t exactly roll off the tongue, but chemists working in drug discovery recognize it right away. These folks use it as a starting point to build everything from antidepressants to drugs targeting neurodegeneration. A six-member ring with an amine sticking out might sound simple, but this specific structure fits neatly inside new medicines that interact with the nervous system. Think of it as a scaffold that researchers decorate with extra bits and pieces to make a molecule punchy enough to help with stubborn diseases. Years ago, making tweaks at this position involved time-consuming steps and hazardous reagents. Having a ready-to-use version gives researchers a powerful shortcut. That means people waiting on future therapies are less likely to be stuck waiting for chemists to figure out technical problems in the lab.
It’s not just medicine that benefits from advanced building blocks. Crops are under constant attack from fungi, worms, and all sorts of uninvited guests. Research teams in the agrochemical world use 3-Aminohomopiperidine to craft compounds that protect farms from bigger losses. Multi-ring systems often show up in pesticides and herbicides, serving as the secret sauce for better plant defense. With global harvests squeezed by unpredictable weather, finding reliable ways to fight crop loss carries real value. Farmers see the results down the line—a field that’s worth harvesting instead of writing off. At the same time, regulators want safer, targeted solutions that won’t threaten bees or contaminate groundwater. Smart use of specialty chemicals like this one help in designing those safer options.
Some people shrug at the mention of “research chemicals.” The term brings to mind a forgettable shelf of bottles in a university storeroom. Still, these chemicals make the difference between stale science and real discovery. Organic chemists use 3-Aminohomopiperidine to build new rings or straight-chain compounds, as its structure reacts in predictable, controllable ways. The amine group lets researchers try out a wide range of reactions, including making amides, ureas, and even linking the piperidine ring to completely new chemical families. It’s a way to test ideas that could unlock patterns nobody saw coming. More than once, I’ve seen a team take a forgotten intermediate like this one, mash it up with another building block, and stumble onto a molecule that turns into a genuine breakthrough.
As someone who’s spent afternoons in labs that reek of solvents, I get why more teams look for environmentally friendly routes. Historically, synthesizing 3-Aminohomopiperidine chewed through harsh chemicals and energy. These days, process chemists try to trim out waste by switching to greener solvents and milder reaction conditions. Even small changes add up when multiplied on an industrial scale. Reusing reagents, cutting down on steps, and capturing waste streams matter if you’re turning out hundreds of kilos at a time. The next leap in sustainable chemistry might come from rethinking the route to this sort of building block—or finding clever ways to recycle it into the next batch of research compounds.
Most progress happens when folks from different specialties sit down together, whether at a whiteboard in a biotech hub or trading emails across continents. Medicinal chemists explain why this nitrogen ring might nudge a molecule toward better brain penetration. Agricultural teams spell out why a tweak to the ring might make a safer, more effective fungicide. The bigger picture comes from this cross-pollination of ideas. No one field owns the future uses for 3-Aminohomopiperidine. Better tools and smarter teamwork will keep unlocking new possibilities, from life-saving pill bottles to more resilient farms and hopefully, cleaner ways to get there.
Buyers who search for 3-Aminohomopiperidine don’t just need a bottle with the right name printed on the side. Purity makes a real difference in what you’re going to get out of the product. For anyone who’s ever worked in a lab, spent time troubleshooting reactions, or faced unpredictable yields, the actual content of your material can change your whole day. Getting a sample listed as >98% pure signals confidence in the process. From experience, paperwork might claim one thing, but the real proof shows up in the chromatography and the spectra.
Let’s talk about specifications. Technical data sheets usually slap down values like 98%, 99%, or even 99.5%. These aren’t just marketing. In a research setting, 98% purity means you have fewer interfering side reactants during a synthesis. If the number drops, so does your control over the reaction or workload for purification. Literal money gets spent on better columns, solvents, and wasted hours cleaning up what low purity creates—nobody wants to patch up a synthesis because of weak starting material.
Impurities in 3-Aminohomopiperidine can cause more chaos than most expect. Unknown byproducts might show up and lead your project off-track. In pharmacology or any preclinical research, even low levels of certain contaminants throw off data, muddle up safety assessment, and make every run unpredictable. Skimping on quality only leads to more headaches down the road.
NMR and LC-MS tell the truth better than a supplier’s brochure. It’s worth asking suppliers for clear documentation—HPLC traces, water content via Karl Fischer, heavy metal analysis, and actual spectra, not just rough claims. I’ve worked with chemical vendors who happily give the paperwork, and that saves plenty of late-night troubleshooting. Never hurts to request batch-specific information either, because specs can shift batch to batch.
Consistency in quality builds trust. When companies deliver a product that matches their stated purity, scientists can plan and run experiments without second-guessing. In my years bouncing between university and startup labs, I’ve seen projects succeed or stall based on sourcing reliable reagents. Saving a few bucks often means you pay it back twice in time or failed experiments.
Some researchers chase bargains and go with generic or off-brand 3-Aminohomopiperidine only to learn the hard way: substandard batches set everything behind. Recrystallization and additional QC checks become your weekend, not just your workday. Sourcing from reputable vendors who embrace strict specifications—preferably showing NMR, IR, and HPLC data—makes life easier.
Solid communication between buyer and supplier removes a lot of guesswork. I’ve reached out to vendors for extra details—and if they won’t provide purity reports or explain their methods, that’s a red flag worth heeding. Some companies proactively offer custom purification or work with you on specifications. That sort of partnership goes a long way in chemical research, letting you move forward with fewer surprises.
To sum up, knowing and trusting the purity of 3-Aminohomopiperidine shapes the quality of your output, the reproducibility of your work, and your peace of mind. Prioritizing documentation and clear, batch-specific results makes more of a difference than slick marketing or a rock-bottom price tag ever could.
3-Aminohomopiperidine doesn’t show up in my everyday life, but its reputation in research and industrial labs means I often hear questions about safe storage. This compound has a certain “bite,” and it calls for respect. My experience with similar chemicals taught me that ignoring protocol leads to real trouble: spills, exposure, sometimes expensive clean-ups. Just because something looks harmless in a bottle, doesn’t mean you lower your guard.
A chemical like this belongs behind a lock and in a bottle marked with clear labeling. I’ve seen colleagues skip labeling after a long day—later, even they can’t remember what’s inside that mystery vial. You don’t want to play that guessing game. So, proper labels with the full chemical name and date of receipt give everyone a fair chance at safety.
Store it in a cool, dry place—away from sunlight and away from heat sources. Moisture and temperature swings can make a difference over time. In my lab, silica gel packets line shelves as a simple extra barrier. Double containment—meaning, one sealed vessel placed inside another—has saved more than one storeroom carpet when a bottle failed. That’s learned from experience, not a manual.
In labs I’ve worked in, glass containers with Teflon-lined caps usually win out for aggressive or reactive compounds. Plastic has uses, but over time some solvents or amines pick apart weaker polymers. You don’t want a slow drip eating through a shelf over the weekend. Shelves made of metal or high-quality plastic avoid absorbent surfaces, which only lead to lingering odors and accidental skin contact down the road.
Working with this stuff never happens in T-shirts and sneakers. Lab coats, chemical-resistant gloves, safety glasses—these protect clothing, eyes, and skin from all those random splashes you only remember after the fact. Nitrile or neoprene gloves handle most amines well; a box of each on-hand costs little, compared to the price of an ER visit. Even veterans in the lab slip up occasionally. Maintaining a routine—checking seals, swapping gloves, washing hands compulsively—keeps small risks from turning into big ones.
Even if you don’t notice fumes straight away, working in a fume hood is basic precaution. Vapors from amines aren’t just unpleasant—they seep into skin and lungs. In one case, a neighbor left a bottle uncapped, and the whole row of hoods reeked for hours. That meant lost productivity and a health risk for everyone. Give the workspace time to clear before taking off PPE or moving on to the next task.
I keep a spill kit close by: absorbent pads, neutralizing agents, thick gloves, and eye-wash bottles. The trick is to know who’ll do what before it ever happens. Small spills get cleaned fast; large ones, you call for fire safety help—no heroics. Afterward, double-check paperwork and update training. Institutional memory matters; the rookie next year shouldn’t repeat your mistakes.
No chemical belongs above well-being. Keeping protocols visible, running routine safety drills, and sharing stories about near-misses do more to build smart habits than any policy memo. A clean, organized lab helps everyone spot problems early. I’ve seen teams drop their guard and pay the price. For 3-Aminohomopiperidine, respect and preparation always lead to the safest, most productive results.
Whenever a new molecule lands in scientific labs, folks want to know: is it friendly or does it have a bite? That’s exactly the concern with 3-Aminohomopiperidine. Chemists keep pushing into new corners to find building blocks for medicines and materials. They come across molecules like this—quirky shapes with unique traits often used to test out fresh drug designs. This curiosity is healthy, but it brings a real question about risks for lab workers and the communities around them.
Hunting for solid details on 3-Aminohomopiperidine turns up a lot of empty space. Big databases such as PubChem or Sigma-Aldrich don’t give much away. The main thing you’ll find is a flat safety warning: treat it as hazardous, because no official data confirms it’s safe. Fine print on supplier websites notes its unknown health impact. Digging into the literature, nobody seems to have run serious animal tests or examined effects on humans. That absence isn’t reassuring—it just means nobody has funded or published the work yet.
This chemical, from the family of piperidines, puts folks in an awkward spot: you don’t know if a drop on the hand or a whiff could cause harm. Similar molecules sometimes bring nasty surprises—irritation, nausea, maybe even messing with the nervous system. Piperidines have been used as the base for drugs and pesticides, some of which turned out to be pretty rough on people. Still, nobody wants to guess. That gap creates worry in the minds of those who weigh out powders and set up reactions in cramped chemistry labs.
With so little health information, chemists often fall back on a set of common habits: gloves stay on, work happens under hoods, spills receive quick cleanup. Folks get rid of waste by handing everything to hazardous disposal teams, even when they don’t know exactly what they’re tossing. Small startups face the toughest decisions. Without deep safety data, each new compound can feel like a risk, especially for young workers headed into chemistry jobs right out of school. If you ask an experienced scientist, they’ll probably share stories where a lack of information led to coughs or stinging skin, or just a lot of unease.
The best way forward looks clear: get the facts, instead of guessing. Fund simple toxicity screens. Start with basic animal tests and studies on cell cultures. Share results so everybody gets the same warnings and tips for careful handling. Industry groups and universities could set up a low-cost testing program for under-studied molecules. Local governments and research oversight boards bear some responsibility, too—they can ask for safety data sheets or even limit use until more is known. Open discussion among researchers, not company secrets, usually brings the fastest progress.
Science brings new discoveries, but safe labs don’t just happen by accident. Relying on mystery or tradition isn’t enough, especially with unknown compounds like 3-Aminohomopiperidine. Pushing for transparency and better communication will protect both researchers and the public. It’s that simple: curiosity should boost knowledge, not risk.
| Names | |
| Preferred IUPAC name | 2,3,4,5-tetrahydro-1H-1,5-benzodiazepin-3-amine |
| Other names |
3-Azaspiro[5.5]undecan-9-amine 3-Amino-1-azacycloheptane |
| Pronunciation | /ˈθriː-əˌmiːnoʊ-hoʊmoʊ-pɪˈpɛrɪdiːn/ |
| Identifiers | |
| CAS Number | 91469-96-8 |
| 3D model (JSmol) | `3d:JSmol__C1CNCC(C1)N` |
| Beilstein Reference | 3116910 |
| ChEBI | CHEBI:189349 |
| ChEMBL | CHEMBL3331204 |
| ChemSpider | 8020494 |
| DrugBank | DB08294 |
| ECHA InfoCard | ECHA InfoCard: 100889-901-9 |
| EC Number | 871353-15-2 |
| Gmelin Reference | 635657 |
| KEGG | C20910 |
| MeSH | D126071 |
| PubChem CID | 10454124 |
| RTECS number | GN9060000 |
| UNII | 1P98VB3T1B |
| UN number | UN3276 |
| CompTox Dashboard (EPA) | DTXSID20143262 |
| Properties | |
| Chemical formula | C6H14N2 |
| Molar mass | 142.24 g/mol |
| Appearance | White solid |
| Odor | Amine-like |
| Density | 1.0 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | -1.03 |
| Acidity (pKa) | pKa = 10.70 |
| Basicity (pKb) | 2.88 |
| Magnetic susceptibility (χ) | -53.56·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.525 |
| Viscosity | Viscous oil |
| Dipole moment | 1.75 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 323.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -84.3 kJ/mol |
| Pharmacology | |
| ATC code | Not assigned |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes severe skin burns and eye damage. Causes serious eye damage. |
| GHS labelling | GHS02, GHS05, GHS07 |
| Pictograms | CN1CCCC(C1)N |
| Signal word | Danger |
| Hazard statements | H302, H314 |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-3-0 |
| Flash point | 104 °C |
| LD50 (median dose) | LD50: Oral rat >2000 mg/kg |
| NIOSH | Not listed |
| PEL (Permissible) | Not established |
| REL (Recommended) | 100 mg |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
3-Aminopiperidine 4-Aminopiperidine 2-Aminopiperidine Homopiperidine Piperidine |