Interest in 3-Acetylpyrrolidine started around the middle of the twentieth century, during a period when chemists were pushing hard to make sense of small nitrogen-based rings. Labs in Europe and the United States poured resources into new synthetic building blocks as advancements in pharmaceuticals drove the hunt for versatile molecular frameworks. Back then, the emphasis rested less on high purity, more on making something that could act as a scaffold for tailoring biological activity. Early published work explored alkaloids and various N-heterocycles, and 3-Acetylpyrrolidine caught notice for its ease of modification and the stability of its pyrrolidine ring. Moving into the 1990s, more complex derivatizations appeared in the literature, fueled largely by combinatorial chemistry. The compound became a staple in chemical catalogs once demand grew for pyrrolidine-based intermediates both in academia and industry.
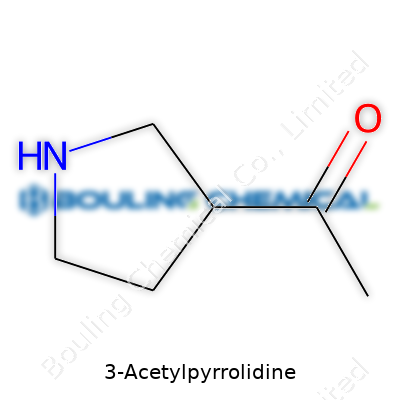
3-Acetylpyrrolidine carries the structure of a five-membered saturated ring with an acetyl group hanging off the third carbon, essentially opening a path for countless downstream transformations. It mostly shows up as a clear, colorless to slightly yellowish oil at room temperature. Commercial suppliers tend to package it in small glass bottles, with users ranging from bench chemists to folks in pharmaceutical research. This molecule doesn’t attract much attention outside specialty labs, but for those who know it, it’s a workhorse—flexible in synthesis, straightforward to purify, and friendly to modifications. I’ve reached for a bottle of it more often than I’d care to admit, especially while working out novel routes to nitrogen-based drugs.
3-Acetylpyrrolidine registers a molecular formula of C6H11NO and weighs in at about 113.16 g/mol. It blends smoothly with most standard organic solvents—methanol, ethanol, ether, even dichloromethane. The boiling point hovers around 190–215°C, though small changes appear depending on atmospheric pressure and impurities. Unlike many related amines, 3-Acetylpyrrolidine avoids the sharp, fishy smell that follows primary amines. The acetyl group boosts its resistance to oxidation and makes the ring less basic, so it resists unwanted side reactions in most build-ups. It doesn’t dissolve much in water, but that rarely poses a problem, since most users deal with organic mixtures.
Suppliers tend to list 3-Acetylpyrrolidine by assay, pointing to purity levels above 97%, and usually throw in a Certificate of Analysis, picking out content of residual solvents and trace impurities. Safety information sticks to GHS standards, and labeling highlights the irritant potential for eyes and skin. Sometimes you see extra details if the batch is destined for regulated industries. In my experience, careful attention to spec sheets proved vital—not every supplier matches quality claims, and trace contaminants often throw off more sensitive applications. Labels tie to barcodes and batch numbers, making it easier for labs to track sources and report issues if something feels off.
The common route starts with pyrrolidine, an inexpensive precursor, made from 1,4-diaminobutane through cyclization. Acetylation at the third position calls for a directed approach—direct Friedel-Crafts acylation won’t cut it on a poor aromatic like pyrrolidine. Labs generally go for lithiation at the third carbon with a strong base like LDA, followed by quenching with acetyl chloride. Yields often float between 70 and 85%, provided conditions stay cold and the reaction vessel remains free from moisture. I recall one winter running batch after batch in a drafty fume hood, only to learn the hard way that water condensing along the neck of the flask can eat away at purity in no time.
Once in hand, 3-Acetylpyrrolidine opens doors to a range of synthetic tricks. The acetyl group pulls electron density, dampening nucleophilicity at nitrogen, which lets chemists carry out selective alkylations, oxidations, or substitutions at ring carbons. Reduction of the acetyl group to an alcohol gives 3-hydroxypyrrolidine, a valuable intermediate all on its own. Cleavage under acidic or basic conditions exposes the primary amine at C-3, a handle for all sorts of amide coupling reactions. I’ve used this compound as a launching pad for chiral catalysts and as a template for pushing new CNS-active agents, where the basicity and size match the needs of pharmacological design.
The name 3-Acetylpyrrolidine shows up in chemistry papers, but you also see it listed as 1-Pyrrolidin-3-yl-ethanone or 3-Oxo-N-ethylpyrrolidine in some catalogs. CAS number 5368-57-6 clears up confusion when ordering. It’s also tagged as Pyrrolidine, 3-acetyl- or N-acetyl-3-pyrrolidine for those searching older literature. Anyone digging through patents or regulatory filings soon learns to check synonyms to avoid missing key safety or synthetic details.
Handling 3-Acetylpyrrolidine calls for gloves, goggles, and access to a fume hood. Spill risks look low, but skin or eye contact causes discomfort, and the safety data sheets warn of respiratory irritation if vapors go airborne. Storage in a cool, dry place keeps it stable over time, but strong acids or oxidizers threaten unwanted side reactions. Waste streams containing this compound should run through organic solvent collection, not down the drain where it could foul up local water systems. Over the years, I’ve watched labmates become careless with even minor intermediates, but a brush with a cloud of vapor prompts quick respect for proper PPE.
Pharmaceutical researchers chase 3-Acetylpyrrolidine for its backbone, serving as a key fragment in CNS drugs, antivirals, and specialty catalysts. It provides a launch point for constructing molecules that target neurotransmitter uptake, with the right polar/hydrophobic balance for brain permeability. Medicinal chemists reach for it while building analgesics, antipsychotics, or antiepileptics; some agrochemical teams look to it for making insecticidal compounds more potent. Its role reaches beyond direct drug candidates, popping up in ligand libraries, robust organocatalysts, and even in flavors and fragrances, where traces of pyrrolidine derivatives bring nutty or cocoa-like notes. Trade shows often display this compound bundled in a suite of N-heterocycles, marketed for the latest combinatorial campaigns in drug discovery.
Labs across the globe tap 3-Acetylpyrrolidine for new molecular scaffolds. The drive for more selective, less toxic therapeutics keeps chemists looking for nitrogen rings that slip past metabolic breakdown and avoid off-target effects. Recent research in asymmetric synthesis taps into its ability to anchor stereochemistry, leading to new generations of enantioselective catalysts and ligands. Multistep syntheses for peptidomimetics, antivirals, or enzyme inhibitors often include this building block, since the ring holds up under a range of reaction conditions. My own projects relied on its durability—few intermediates survive both strong acids and bases, but this one can weather the storm.
Despite its routine use, official data on human toxicity remains sparse. Rodent studies hint at moderate oral and inhalational toxicity, though the compound rarely accumulates in tissue thanks to the metabolic lability of the pyrrolidine ring and the ease of acetyl cleavage. Chronic exposure raises more questions, with longer-term analysis still underway to clarify carcinogenic or mutagenic potential. I’ve spoken with toxicologists who urge caution for prolonged or repeated use—just because no immediate effect appears doesn’t guarantee absence of risk. More studies are needed, and tighter regulation may follow as new pharmaceuticals and pesticides roll out using this scaffold.
As pharmaceutical development turns toward more targeted, personalized medicine, 3-Acetylpyrrolidine stands out for flexibility and ruggedness. Blockbuster drugs keep shifting toward N-heterocyclic scaffolds that absorb and clear quickly, and this compound fits the bill. Meyer’s group at the University of Vienna recently outlined several new derivatives for next-generation CNS agents, all hinging on modification at the acetyl group. Expect broader use in green chemistry, too, as demand grows for smaller, lighter, more easily recycled building blocks. With the relentless pace of chemical innovation, 3-Acetylpyrrolidine’s future looks tied to inventiveness—how researchers exploit its chemistry and how regulators respond to growing real-world exposure. My bet is that for years ahead, this quiet, versatile cyclic amine will keep finding a seat at the table whenever tight, selective synthesis matters.
There’s something a little satisfying about the clean numbers behind a chemical name. 3-Acetylpyrrolidine keeps things simple but interesting. The molecular formula—C6H11NO—packs some meaning. Every time I see an acetyl group and a pyrrolidine ring in one molecule, it reminds me how a few changes in structure spin into different properties.
C6H11NO stands for 6 carbons, 11 hydrogens, 1 nitrogen, and 1 oxygen. Lay it out, and suddenly the shape of that molecule starts to take hold in your mind. The molecular weight comes in at 113.16 g/mol. That number isn’t just for the label; it's carried into labs and manufacturing. Chemists work out pure quantities, lab techs weigh out reagents; everywhere, precision keeps mistakes in check.
Working with small molecules often felt like solving a puzzle. Forget a single hydrogen or misread a formula, and results skew fast. I once tried to scale up a similar compound in a basic university lab. At one point, a colleague had miscalculated the amount needed by just 1 g/mol. The synthesis yield tanked. Accurate formulas and weights steer researchers toward the right path, especially for pharmaceuticals or industrial intermediates.
3-Acetylpyrrolidine doesn’t usually headline science news, but that’s missing the forest for the trees. It sits in the families of molecules that feed into drug development, agrochemicals, specialty polymers. Firms often build larger molecules off cores just like this. Getting formulas and weights wrong costs real money and slows down projects.
In recent years, as chemistry moved into green and sustainable trends, knowing the molecular details allows companies to tighten up what goes in and what comes out. Waste reduction starts with simple math and reliable compounds. I’ve seen startups obsessed with record-keeping, tracking every batch. Regulatory compliance, environmental safety—all hang on formulas and weights as basic infrastructure.
Mistakes don’t usually happen because someone is careless. It’s easy to copy a formula straight off a datasheet and still miss something: suppliers make typos, translation errors sneak in, or an old IUPAC name leads to confusion. Running tests, cross-referencing with public chemical databases like PubChem or ChemSpider, and physically checking molecular weights with mass spectrometry or NMR bridges the gap between theory and what arrives in a bottle.
I’ve found the most consistency working with digital lab notebooks that double-check chemical inventories against updated databanks. The field has moved away from scribbling on loose paper—a relief. If a formula changes after a regulatory reclassification, at least users can see updates instantly. Building quality assurance into the workflow makes a difference, too, especially on tight timelines where errors quickly snowball.
C6H11NO and its 113.16 g/mol weight are more than textbook numbers—they’re golden metrics for anyone who wants to avoid costly reruns, hazardous mixing, or regulatory fines. Chemistry builds on naming things for a reason, but it pays to check, double-check, and lean on up-to-date resources. Every pipette, every reaction flask, every manufactured product downstream owes something to starting with the right numbers.
If you talk chemistry in a room full of folks, 3-Acetylpyrrolidine probably won’t spark instant recognition. It never landed on the trending list, but behind lab doors and in technical spaces, this compound carries more weight than its five-carbon skeleton might suggest. I’ve sat at project tables where it stood out for its versatility in research and manufacturing.
I’ve worked with teams that needed a certain backbone structure to move a project pipeline forward. One place 3-Acetylpyrrolidine often steps in is the pharmaceutical world. Here, it pops up as an intermediate, not as a finished pill, but as a stepping stone. Drug development doesn’t happen in a straight line; it’s a relay race where each handoff needs a reliable runner. This compound provides a framework that helps chemists build a range of drugs, from antivirals to nervous system agents. Without intermediates like this, synthesizing new molecules eats up double the time, and resources thin out fast.
I’ve seen research grind to a halt waiting on a key molecule, and 3-Acetylpyrrolidine can save the day, especially in agricultural chemistry. Life in this field is all about optimization—effective pest control, safer herbicides, more efficient plant protection. This compound supports synthesis steps for many agrochemical products. Farmers might not see it, but they feel its impact through more resilient crops and improved yields.
The food industry draws on a different side of the compound. In flavor chemistry, small tweaks in molecular structure lead to big shifts in taste and aroma. Food labs use molecules like 3-Acetylpyrrolidine to design and test new additives. Sometimes, a molecule plays a supporting role, steering the reaction toward more appealing flavors. That’s a behind-the-scenes job that shapes the end product more than the average shopper realizes.
Walk down the aisle of any electronics store, and you’ll spot devices made from advanced polymers and specialty materials. Chemists invested in new battery technologies, coatings, or plastics sometimes rely on heterocyclic compounds in their experiments. 3-Acetylpyrrolidine fits the bill because of its amenable structure and reactivity. It helps adjust the properties of new materials, whether that means improving thermal stability or adding flexibility. That sort of chemical tinkering turns future concepts into usable products.
In my time working with startup incubators, creativity often came down to having the right building blocks on hand. University and industrial researchers explore 3-Acetylpyrrolidine not for what it does alone, but for how it pushes boundaries in synthesis. By swapping out a single atom or modifying a group, entirely new molecules emerge. Each tweak opens the door to fresh rounds of testing, with hopes of stumbling onto a breakthrough in disease treatment or green chemistry.
One headache many labs run into is reliable sourcing at a fair price. Fluctuations in supply or purity create hold-ups. Increasing transparent production and sharing best practices among suppliers would go a long way here. I’ve also seen how green chemistry movements push for cleaner, safer synthesis methods. By cutting down on waste and hazardous byproducts, we not only protect workers but also open doors for more industries to use 3-Acetylpyrrolidine responsibly. Collaboration between academic groups, manufacturers, and regulators can close this gap.
Every lab tech or chemist who’s ever worked with specialty chemicals knows a small error in storage can ruin a good batch or put teammates at risk. 3-Acetylpyrrolidine isn’t one of those notorious substances you find in every undergrad teaching lab, but ignoring basic storage attitudes with it guarantees headaches down the road.
On the shelf, 3-Acetylpyrrolidine won’t explode at a dirty look, but it rewards care and attention. The bottle sits best in a spot with cool temperatures—something steady below 25°C gives everyone peace of mind. Fluctuating heat makes many small molecules push toward breaking down or reacting with moisture in the air. I once saw a colleague store a small vial near a sun-facing window in a student lab and a crusty, yellowish film appeared within a week. There’s nothing like pitching a ruined container to make you appreciate clear inventory rules.
Sunlight isn’t just for houseplants; chemical stocks need it kept away. Ultraviolet rays from direct sunlight can drive odd transformations, sometimes slow, sometimes dramatic. Stashing the bottle in a dark cabinet or an amber glass container blocks most of that trouble.
No chemist likes sticky screw caps. A tight seal using a screw cap with a gasket keeps out air, which brings moisture and sometimes oxygen. Pyrrolidines in general don’t always love water. That’s why laboratories keep desiccators handy, especially over the summer when ambient humidity spikes. If your shelf lives in a swampy region, using a desiccant pack near your bottle shuts down one more possible headache.
Mixing up labels on reagents has started more stories in research than anyone likes to admit. A bold label, plus an updated date, helps the next user avoid mistakes. A friend once mixed reagents in a rush and had to redo a week’s work. It sounds simple, but these steps are often skipped in a real-world rush.
Chemicals don’t appreciate close company all the time. I always recommend storing 3-Acetylpyrrolidine away from acids and oxidizing agents. It may not turn into a fireball, but funky products may arise, and cross-contamination can foul up experimental results. Strong-smelling chemicals and solvents sometimes bleed into each other’s containers too. Giving each family of chemicals its own cabinet saves a lot of grief later. This lesson comes straight from spending months in academic stockrooms where one open flask can turn a cabinet into a paint-thinner carnival.
Gloves and safety glasses are your friends. 3-Acetylpyrrolidine can irritate skin and eyes if spilled. Wearing long sleeves or a lab coat keeps drops off your skin, and a fume hood is important if you open it for any length of time or on a big scale. I’ve noticed people sometimes skip goggles for “quick” work, but splashes don’t wait for lengthy tasks.
Spill kits and good ventilation represent real-world ways to handle surprises. Too many labs stash spill-neutralizing powder in a back corner. Keeping it actually reachable makes a difference in a pinch.
Taking a careful approach to storage and handling of 3-Acetylpyrrolidine isn’t bureaucracy—it’s how you keep work safe, results consistent, and budgets intact. From the right darkness and dryness to smart separation, a few smart habits always save trouble. Even chemicals that seem low-danger reward respect and some old-fashioned common sense in the lab.
3-Acetylpyrrolidine isn’t a household name, but folks in chemistry, research labs, or certain industries know it well. The compound sits on shelves in glass bottles, often with a firm warning sticker. You can spot it by a slightly fishy odor and a clear to yellowish liquid look. It’s used mostly for synthesizing more complex chemicals, pharmaceuticals, and as an intermediate in organic reactions.
Here’s the reality—many organic compounds, especially heterocyclic amines, can cause trouble. 3-Acetylpyrrolidine may irritate the skin, eyes, and lungs. I’ve seen someone get a little careless pipetting a similar compound without gloves; red, itchy hands followed quickly. Vapors, if not contained, could sting the nose and cause coughing or dizziness.
There’s also a fire risk, since it’s a flammable liquid. Pouring or heating it near hot plates will raise eyebrows for good reason. And like most small-molecule organics, accidental swallowing or splashing in the eyes leads to emergency procedures you won’t soon forget. The Material Safety Data Sheet (MSDS) spells all this out, but in practice, people sometimes skip reading it. That’s where trouble starts.
In my own time working in academic labs, I picked up a firm respect for anything with a warning symbol. The small stuff can pack the biggest punch. For 3-Acetylpyrrolidine, exposure limits remain poorly defined; it’s not as notorious as benzene or formaldehyde, but the room for error remains slim. Chronic exposure studies lack detail, which makes me cautious—unknown risks can be the worst kind.
A case years back involved ventilation issues during a winter storm. Hood airflow dropped, fumes built up, and within minutes, headaches and dizziness crept in among those working late. Only after stepping outside did folks realize how quickly vapors can sneak up, even with what seems like routine handling. After that, everyone checked airflow dials and took hood alarms seriously.
Good habits beat luck, every time. Before working with 3-Acetylpyrrolidine, I pull up the data sheet, check compatibility of gloves, and make sure my goggles are clean. Not all gloves block organics; nitrile usually works, but double-checking never hurts. Splash goggles guard against unexpected squirts or bursts, especially when handling pressurized containers.
Fume hoods should always be running full blast. Open bottles only inside an enclosure where a slight negative pressure keeps vapors away from the nose. Keep spill kits nearby—spills shouldn’t become a scramble. Wash stations and eye flushes should work and be within reach. Scrambling for the key to a bathroom after a spill doesn’t cut it—speed matters.
Labeling matters more than people think. Once I found a clear liquid in a beaker with just a handwritten “A.” Who knew if it was acetone, ether, or something nastier? Proper labeling, clear protocols, and communication cut down on accidental mixing or unexpected exposures.
For better protection, labs and workplaces should keep training sessions regular. New hires, students, and old timers alike benefit from reminders—safety isn’t just a box to check. When new chemicals join the shelf, everyone needs to know what’s changing. Automated ventilation monitors and tighter inventory controls have helped some labs catch leaks or misplaced chemicals before real problems start. Software for chemical management makes it easier to keep track of storage locations, expiration dates, and who used what. Taking these extra steps reduces near-misses and steers the team clear of unpleasant surprises. If a place handles 3-Acetylpyrrolidine regularly, building safety into the workflow keeps everyone healthy and out of trouble. That approach beats cleaning up accidents any day.
3-Acetylpyrrolidine isn’t a chemical you find on every shelf. Most researchers, especially those in pharmaceuticals or advanced material synthesis, care a lot about purity. Watching colleagues struggle with inconsistent results taught me that lower grades in fine chemicals often multiply the chance of redoing experiments. Purity with 3-Acetylpyrrolidine tends to drift toward 97% or higher, sometimes up to 99%. Years back, a buddy running a medicinal chemistry lab learned the hard way that the remaining few percent can mean stubborn impurities that spoil an entire synthesis step. That 2-3% can jam up a column or spark unplanned side reactions, adding another layer of unpredictability to work that already borders on the unpredictable.
Supplier catalogs show their hand fast: the higher the purity, the steeper the price. Lab stand-bys like Sigma-Aldrich, TCI, and Alfa Aesar keep it simple with their listings, though sometimes you need to email a technical rep to pin down if a batch is closer to 97% or flirting with 99%. Even for universities or industrial labs willing to pay a premium, there are headaches if the grade listed doesn’t match what's in the certificate of analysis. That’s earned plenty of suppliers a side-eye over the years.
Availability almost always depends on where you stand. In the US, most chemistry departments or biotech startups check with a handful of major suppliers. They tend to carry small quantities – we’re talking grams or tens of grams. Turn to China or India, and wholesale suppliers often offer kilos, sometimes labeled only as “technical” or “research” grade. Plenty of customers, though, hesitate when the paperwork trails off or shipping times drag past customs inspections. I once saw a four-week delay domino into a project setback because customs flagged an ambiguous SDS sheet.
Batch size can dictate purity. Some manufacturers scale back scrutiny when pumping out larger orders, which leaves inconsistencies. Buyers in drug development circles swap notes on this problem a lot: order small, and purity sticks high and reliable; go big, and you’re betting on the supplier’s quality controls holding up.
The scramble for purity and availability brings up practical fixes. One big step forward: third-party batch testing. That extra layer of confirmation turns a lot of supplier ambiguity into a straight answer. Some groups I know pitch in and share independent analysis before ordering, especially on social chemistry forums or closed buyer’s groups. Distributors slowly catch on—more reports, tighter specs, and better batch tracking show up in their statements now.
Regulations kick in, too. Europe’s REACH and the US TSCA programs nudge suppliers to clean up documentation, which helps everyone from university labs to private R&D shops. Still, the fastest fix is better transparency from suppliers. Only so many scientists have the budget to pay twice for a bottle just to verify its contents. Pressure from big customers forces some improvement, but smaller labs still dodge surprises by calling for references, previous batch certificates, and peer recommendations.
Reliable access to high-purity 3-Acetylpyrrolidine fuels everything from drug discovery to advanced polymer projects. The work gets smoother if buyers and sellers both keep honesty on the table. Until then, the smart play rides on independent verification and sharing hard-won experience in the community.
| Names | |
| Preferred IUPAC name | 1-(Pyrrolidin-3-yl)ethan-1-one |
| Other names |
1-Acetylpyrrolidine Pyrrolidine, 3-acetyl- 3-Acetyl-1-pyrrolidine |
| Pronunciation | /ˈθriː əˈsiːtɪl pɪˈrɒlɪdiːn/ |
| Identifiers | |
| CAS Number | 765-43-5 |
| Beilstein Reference | 1718736 |
| ChEBI | CHEBI:77186 |
| ChEMBL | CHEMBL3210495 |
| ChemSpider | 79508 |
| DrugBank | DB04236 |
| ECHA InfoCard | 100.105.822 |
| EC Number | 214-306-9 |
| Gmelin Reference | 94447 |
| KEGG | C19139 |
| MeSH | D018105 |
| PubChem CID | 11966278 |
| RTECS number | UF5605000 |
| UNII | 6U7XEZG7CF |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C6H11NO |
| Molar mass | 113.16 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | amine-like |
| Density | 0.997 g/cm3 |
| Solubility in water | Soluble in water |
| log P | 0.01 |
| Vapor pressure | 0.4 mmHg (at 25 °C) |
| Acidity (pKa) | pKa = 11.27 |
| Basicity (pKb) | 2.94 |
| Magnetic susceptibility (χ) | -44.53·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.4780 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.73 Debye |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 345.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -3626.8 kJ/mol |
| Pharmacology | |
| ATC code | |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | Precautionary statements: "P261, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 93 °C |
| Autoignition temperature | 310 °C |
| Lethal dose or concentration | LD₅₀ (oral, rat): >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): 670 mg/kg (rat, oral) |
| NIOSH | KJ7U56E2OZ |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.2 mg/m³ |
| Related compounds | |
| Related compounds |
2-Acetylpyrrolidine 1-Acetylpyrrolidine 3-Formylpyrrolidine 3-Acetylpiperidine 3-Acetylpyridine |