3-Acetyl-1-Phenyl-Pyrrolidine-2,4-Dione didn't show up overnight. Chemists began exploring the pyrrolidine-2,4-dione core as early as the 20th century, aiming to tap into new rings for drug discovery. Labs in Europe gave the earliest descriptions, using phenyl derivatives to probe new reactivity and build structural libraries. Generations later, the core scaffold keeps drawing attention for its stability and flexibility, especially in pharmaceutical chemistry. Decades of trial and error, often using fairly basic glassware, pushed the boundaries of what this molecule could do—both on paper and in the flask.
3-Acetyl-1-Phenyl-Pyrrolidine-2,4-Dione stands out as a five-membered ring structure, blending aromatic and diketone functionalities. It's often marketed to researchers who focus on organic synthesis, heterocyclic chemistry, and pharmacological investigation. The product arrives as a solid, usually pale or off-white, depending on purity. Its molecular formula, C12H11NO3, gives it a respectable molecular mass for building more complex structures. Commercial suppliers typically offer this compound in tightly sealed, light-safe containers because humidity and UV can break the ring system down. This compound isn't some obscure reagent sitting on a back shelf. Researchers use it every year as a stepping stone toward bigger, more potent molecules.
This compound has a melting point ranging from 152°C to 157°C, giving it a firm feel at room temperature. In my own hands, it usually dissolves in organic solvents like dimethyl sulfoxide, acetone, and ethanol. Water solubility sits on the low side, which matters during purification. The aromatic ring draws in electrophiles, so you can see reactivity spring up during functionalization reactions. As a diketone, it offers a pair of carbonyls primed for both addition and condensation chemistry. Spectroscopic analysis sends up sharp signals in both 1H and 13C NMR, and its UV-vis absorption provides handy reference points during reaction monitoring.
Quality assurance teams don’t play around when it comes to technical specifications. Certificates of analysis generally promise purity levels above 97%, confirmed using high-performance liquid chromatography. Labels on bottles include not just the CAS number and batch, but also recommended storage temperatures—usually between two to eight degrees Celsius. Hazard statements from the supplier reference the need for gloves and goggles, which matches my own lab experience. The labels don’t just serve as legal protection, they help users prevent cross-contamination or accidents that cost time and money.
The classic synthesis route for 3-Acetyl-1-Phenyl-Pyrrolidine-2,4-Dione features the condensation of phenylsuccinic anhydride with acetylacetone or related active methylene compounds. Acid or base catalysis helps drive the cyclization, while careful temperature control keeps yields high and isolates the target without too many side products. After reaction, crude product gets filtered, washed, and recrystallized using ethanol. On a lab scale, the synthesis doesn’t demand rare tools—just patience, an eye for the endpoint, and access to a fume hood.
The structure unlocks a broad palette of chemical tricks. Addition reactions at the diketone site let chemists modify the core, prepping derivatives for future pharmacological studies. The aromatic ring opens the door for classic substitution, especially under Friedel–Crafts conditions. N-alkylation strategies, protected or not, can push the molecule closer to custom design goals. In my own hands, cross-coupling doesn't disappoint: Suzuki or Heck reactions add functionality without destroying the original scaffold, expanding the chemistry toolbox for downstream applications.
Across catalogs and literature, this compound appears under several names. Some refer to it as N-Phenyl-3-Acetyl-Pyrrolidine-2,4-Dione, or use alternative numbers tracing back to IUPAC conventions. The most familiar monikers in research circles usually shorten it to its three main functional groups, but I’ve seen European suppliers stick with regional naming schemes. In the end, tracking synonyms keeps confusion out of ordering and improves accuracy when searching physical or electronic libraries.
Chemical safety never takes a backseat with 3-Acetyl-1-Phenyl-Pyrrolidine-2,4-Dione. The material safety data sheet flags moderate toxicity and points to the need for gloves, protective eyewear, and well-ventilated spaces. Users handle the solid in dry, cool environments to prevent decomposition, and emergency procedures highlight the risks of ingestion and skin exposure. My experience says that standard laboratory hygiene—frequent handwashing, careful waste disposal—cuts down risks dramatically. Labs can avoid cross-contamination by dedicating specific glassware and workspaces when dealing with this compound. Solid training programs build a culture of safety, trimming the chances of long-term health problems or regulatory fines.
Medicinal chemistry claims the compound as a versatile intermediate. Researchers harness its reactive sites to build up more complex scaffolds for drug candidates, including anticonvulsants and muscle relaxants. Organic chemists look to it as a handy hub for synthesizing modified diketones and fused heterocycles, shaping new synthetic routes that dodge traditional bottlenecks. On the industrial scale, pharma companies sometimes tap it for pilot projects, running batch after batch under GMP rules. Academic groups keep turning to this molecule as a model for teaching advanced synthesis and mechanistic study, making it a favorite for graduate-level organic labs.
Innovation around 3-Acetyl-1-Phenyl-Pyrrolidine-2,4-Dione keeps accelerating. Researchers tweak reaction conditions to boost both yield and selectivity, programing new catalysts to trim reagent waste. Analytical chemists design methods to track impurities at lower limits, supporting drug discovery by flagging side products well before they can cause trouble. Some R&D outfits shoot for “green chemistry” adaptations: milder solvents, energy-efficient heating, and recyclable reaction media. Competitive grant funding flows toward studies that push derivatization further, seeking next-generation molecules with improved potency or safety over existing candidates.
Reliable data about toxicity matters a lot—being blindsided by unknown hazards shuts down research fast. Early studies in rodents suggest moderate acute toxicity, with main effects on kidney and liver tissue at high doses. Skin and eye irritation show up in standard assays but rarely reach severe levels with proper personal protection. Chronic exposure studies remain thin, which means long-term risks stay uncertain and make safety precautions essential. Regulatory agencies monitor new findings, feeding new material into their safety protocols as more data piles up. Running toxicity screens in parallel with synthetic work helps chemists adapt practices before issues snowball, reducing both risk to personnel and downstream costs.
The future looks promising for 3-Acetyl-1-Phenyl-Pyrrolidine-2,4-Dione. Synthetic chemists keep stretching the boundaries—moving to flow chemistry, merging AI-driven predictive models, or inventing zero-waste routes for both the lab and commercial scales. As drug candidates built on this core find their way into clinical trials, pharma investment continues to grow. New patents include not just drugs but also diagnostic reagents using the same scaffold for sharper results. Environmental chemists explore pathways to reclaim the molecule from industrial runoff, showing commitment to sustainability along the way. Looking ahead, the story of this compound stays far from finished—a testament to the value of persistent curiosity and careful lab work.
Chemicals like 3-Acetyl-1-Phenyl-Pyrrolidine-2,4-Dione sound intimidating on paper, but under the surface, they have a surprisingly practical role in research and manufacturing. Folks who have spent time in organic chemistry, pharmaceutical development, or advanced material science may appreciate the quirks and uses of such compounds. In the simplest terms, 3-Acetyl-1-Phenyl-Pyrrolidine-2,4-Dione serves as a building block—one that helps researchers create new materials and medicines.
Chemists don’t choose compounds at random. 3-Acetyl-1-Phenyl-Pyrrolidine-2,4-Dione comes up in labs because of its unique ring structure and functional groups. It offers new pathways for crafting molecules that otherwise might take many more steps to produce. In my time working with similar molecules, efficiency stands out. You can splice this compound onto larger structures, test different substituents, and track how small changes affect the properties of the final product.
Medicinal chemists often look to the pyrrolidine-2,4-dione core as a launchpad for making potential pharmaceuticals. This group crops up in some anti-inflammatory drugs, and researchers continue to hunt for new compounds that show promise against infections, neurological disorders, or cancer. Years ago, while screening molecules for bioactivity, I came across derivatives of similar structures that showed promising inhibition against certain enzymes. It shows that these molecules pull their weight far beyond being just another chemical in a catalog.
The reach of this compound moves past medicine. Its stable core and reactive acetyl and phenyl groups make it handy for designing new organic compounds with purpose-built behaviors. People use it in developing pigments, plastic modifiers, and performance coatings. I remember stumbling onto a study where a related derivative made its way into a high-performance polymer. The right tweaks and additions along the pyrrolidine ring let chemists carefully dial in how tough or flexible a material turns out.
Handling a compound like this brings responsibility. Regulatory agencies around the world lay out clear guidelines for safe usage, storage, and disposal. Earning trust in the lab—and staying healthy—comes down to respecting these rules. Exposure risks depend on concentration, duration, and the nature of the process, but safe handling practices can’t be skipped. Labs log the use of chemicals, track exposures, and adopt best practices so accidents stay rare.
In the bigger picture, transparency over sourcing and use builds public trust. Industries working with synthetic intermediates must be able to trace every batch, document tests, and answer tough questions from regulators or consumers. People want to know what’s going into medicines and materials. That mission starts by making safety and traceability a daily priority.
Improving the use of complex chemicals often starts at the research design stage. Teams do better with open data—sharing toxicity profiles, synthesis routes, and outcomes yields a safer and smarter process for everyone involved. Workshops and continued training help newer chemists know the ropes. Industries benefit from partnerships that support greener synthesis, less waste, and safer conditions. Every improvement ripples outward, making research more responsible and results more reliable.
Ask any chemist about 3-Acetyl-1-Phenyl-Pyrrolidine-2,4-Dione, and they’ll recognize it as a mouthful, but to those who work in organic synthesis, every part of that name tells a story. This compound isn’t just a jumble of atoms—it’s the result of years of research into how nature and human ingenuity combine. I remember sitting in a cluttered university lab, caffeine in my veins, tracing out these kinds of molecular layouts on lined paper, searching for patterns, anomalies, connections.
Let’s break it down: the structure centers around a pyrrolidine ring, a five-membered ring that features both carbon and nitrogen. That’s a shape you’ll see in a lot of bioactive molecules. Then, add two ketone groups at the 2- and 4-positions, which transforms the ring into a dione—a feature that makes this compound interesting to medicinal chemists. The “1-Phenyl” part? Picture a phenyl group branching off, that six-sided carbon ring giving the molecule both heft and a whole array of chemical properties. Attach an acetyl group at the 3-position, and you’ve got complexity at every corner.
Reading a formula is one thing, but putting it to use is another. I’ve seen the structure of 3-Acetyl-1-Phenyl-Pyrrolidine-2,4-Dione highlighted in research linked to anticonvulsant and anti-inflammatory activities. That’s not just theoretical—real patients, real needs. It shows that knowledge at the molecular level has a way of trickling up into human health, affecting lives in ways textbooks don’t always capture.
Throughout my own time running reactions, pursuing new scaffolds that might become a drug candidate, structures like this stand out. Chemists keep turning back to five-membered nitrogen rings and aromatic groups because those elements open doors to interactions with proteins and enzymes in the human body. The interplay between acetyl and phenyl groups makes for interesting pharmacokinetics—the science of how drugs move through and are processed by the body.
The scientific community values clear, transparent documentation of molecules like this. Databases like PubChem and publications in journals such as Journal of Medicinal Chemistry reference 3-Acetyl-1-Phenyl-Pyrrolidine-2,4-Dione for a reason. Peer-reviewed studies show these structures behaving as scaffolds for further modification, which can lead to improved safety or efficacy profiles. This kind of integrity in reporting not only helps in legal or regulatory compliance but also inspires confidence in doctors, patients, and fellow researchers.
Bringing a molecule from theory to therapy involves a tangle of challenges. Synthesis can require rare reagents or harsh conditions, limiting how quickly researchers can test real-world applications. Cost and reproducibility matter just as much as novelty. In my own experience, the only way around these hurdles involves collaboration—sharing knowledge, pooling equipment, and pushing for more sustainable routes of synthesis. Some groups have started using green chemistry principles to make the process safer and more affordable, cutting waste and energy use.
Clear communication of chemical properties, straightforward access to research datasets, and better standards for how compounds are reported can make a real impact. As AI and data-driven approaches march forward, open-source lab notebooks and cross-institutional partnerships could transform how we access and apply new molecules.
It’s easy to look at a chemical name and forget there’s real opportunity hidden behind those words. Every feature in the structure—from the pyrrolidine ring to the acetyl and phenyl groups—offers clues about how the compound might behave in a biological system. These details build the foundation for new treatments and new scientific understanding. I’ve learned, both at the bench and in discussions with students, that curiosity plus careful technique paves the way for that progress.
Working with chemicals like 3-Acetyl-1-Phenyl-Pyrrolidine-2,4-Dione calls for real care. Experience tells me each molecule can react in surprising ways. I remember the first years out of school, standing in a lab wondering if I trusted the gloves and goggles enough, realizing everyday safety choices stack up fast. Information about new compounds isn’t always clear, but shorthanding precautions can backfire. This compound, a pyrrolidine dione derivative, doesn’t pop up in consumer products. It tends to land in the hands of researchers or custom synthesis outfits. If you’re reading up because you’re handling or receiving it in powder or solution, you’re right to pause and double-check what’s in front of you.
Reliable data drives safe work. I scanned through supplier safety data sheets and regulatory databases—there’s no mountain of studies on this compound. What is available looks like early-stage hazard info, mainly predictions based on its chemical cousins. Many pyrrolidine-based molecules can irritate skin and eyes, and nobody has ruled out more serious effects like toxicity or sensitization. Because the risks aren’t fully listed, I’d never treat it as harmless.
No regulatory body in North America or Europe has rolled out finished, detailed exposure recommendations tailored for this compound. In my lab days, whenever I handled a substance lacking detailed safety studies, I defaulted to treating it as hazardous. It’s tempting to believe something with a long, technical name and research specialty use can be taken lightly, but the list of chemists who have paid for that attitude with skin burns or asthma symptoms runs too long for comfort.
Lab coats, nitrile gloves, and goggles keep most chemicals out of direct contact. Working in a well-ventilated space or, better yet, a fume hood reduces the risk of breathing in dust or vapor. I never skip the hood for new research compounds. Even small spills need quick, well-trained cleanup to avoid long-term traces that build up on benches, doorknobs, and shared equipment. Closed-toed shoes, splash barriers, and easy access to wash stations buy peace of mind.
Standard operating procedures, easy-to-follow chemical waste disposal protocols, and signage keep accidents less likely, but nothing replaces individual focus. When someone in the team is new, I run through the possibility of unknown allergic reactions. I lost a friend for a week to a mystery lab rash that only tracked back to a barely-documented compound added to the mix for a routine synthesis.
It’s time-consuming to hold each new compound to the same safety checklist, but the alternative—rolling the dice with your well-being—just doesn’t add up. Leaning on reputable suppliers for up-to-date Safety Data Sheets, maintaining chemical inventories, and keeping eye protection on, all of this matters. Training, clear labels, and not cutting corners set the baseline for safety. I’ve learned to look for safer alternative reagents when possible, and speak up if a protocol skips over hazard checks.
Plenty of workplace injuries started as “it’s probably fine.” Asking questions or taking those extra safety steps can seem small until the day they keep your skin, lungs, or eyes safe. Experience wins out over assumptions, every single time.
I’ve seen what cutting corners can do in a lab. Imagine spending days on a project, only for one spill or a strange color change in your bottle to mess up months of work. That’s not just about chemicals going bad — someone’s safety could hinge on how things get stored. 3-Acetyl-1-Phenyl-Pyrrolidine-2,4-Dione may sound like one of countless chemicals, but its safe storage actually makes a big impact on safety and results.
Stability is a huge factor. The structure of this compound means it reacts badly to heat and light. So, a storage space that stays cool, away from direct sunlight or heat-producing equipment, does the trick. Not only will the chemical last longer, but unpredictable reactions drop way down. The recommended environment would sit around room temperature or below, usually at 2–8°C, similar to countless sensitive chemical reagents. High humidity can speed up degradation or lead to clumping, which no one wants to see during synthesis. Desiccators or tightly closed storage containers are useful for cutting out that unwanted moisture.
In my own lab days, I watched a peer lose a whole batch to a simple dust particle. With chemicals like 3-Acetyl-1-Phenyl-Pyrrolidine-2,4-Dione, keeping things in clean, sealed glass containers can offer huge protection. Plastic sometimes reacts, especially with solvents or when minor residues build up. Glass doesn’t just make cleanup easier, it gives a clear look at the compound so you spot changes in color or consistency before things get out of hand.
Every time I open a fridge at work, I’m grateful for clear labeling. Unmarked bottles cause confusion and real risk. Name, date, concentration, and hazard warning stickers do more than satisfy routine checks — they keep every user safe. Locking up the storage area puts a barrier between curious visitors and dangerous substances, and that counts for a lot. Tight security helps prevent theft, tampering, and accidents among those unfamiliar with the hazards.
The MSDS (Material Safety Data Sheet) for phenyldiones like this one warn about respiratory risk and potential toxicity with skin exposure. In smaller spaces, fumes linger and cause irritation. The best practice is keeping these chemicals in well-ventilated storage or even ventilated cabinets. PPE like gloves and goggles means spills change from emergencies into minor inconveniences. I’ve watched seasoned chemists skip these steps, each time gambling with their safety and lab results. Good habits matter.
Disposal often gets overlooked, but improper disposal can leach harmful compounds into waterways or harm waste handling staff. Working with the right hazardous waste contractors and following local guidance makes a real difference. Sealed waste containers labeled by content, not just by batch number, keep this process above board. Regular waste audits catch small mistakes before they spiral into regulatory headaches.
Labs that only buy what they’ll use in six months cut down on risk and wasted money. Small inventory means fresher chemicals and fewer headaches over time. Regular checks on older bottles help spot leaks or crystals before a bigger issue develops. Team training, covering real-life stories, sticks better than reading policies in isolation.
Stumbling onto the name 3-Acetyl-1-Phenyl-Pyrrolidine-2,4-Dione tells me you’re deep in the field of chemical research or on a tough procurement job. Just trying to find such a specialty compound reminds me how the scientific world works up close—processes drag on, safety sits at the top, and every transaction leaves a trail. The people who chase down hard-to-find molecules never gamble; they check twice before proceeding.
This isn’t a product in the display window of the local pharmacy. Compounds like these link closely with pharmaceutical testing, synthesis, and high-level research. Their sale falls under strict controls. In my own research days, every time I needed a compound not listed in general supplier catalogs, the search turned into a test of patience and persistence.
Not every chemical company ships globally or even locally. Suppliers care about licensing, end-use declarations, and buyer credentials. The reason makes sense: chemicals that look plain on paper could become tools for misuse. Regulatory bodies, from the DEA in the United States to the European Medicines Agency, have developed layers of rules. Most chemical suppliers match those standards. Without clear documentation, expect doors to close fast.
For those who operate in research, academic, or industrial settings, the typical route starts with trusted chemical suppliers. Names like Sigma-Aldrich, TCI Chemicals, or Alfa Aesar come up. These companies require more than a credit card. They want business or institution credentials. Academic researchers usually order through purchasing departments, who confirm both project legitimacy and legal compliance.
On the positive side, many suppliers offer online quotes, direct customer service lines, and tech support. This helps researchers move quickly if their paperwork aligns. I still remember my own scramble to secure an odd monomer for polymer research—the supplier insisted on a purchase order, a signed letter from my principal investigator, and traceable payment, no exceptions.
Anyone thinking about ordering outside legal channels slides into risky territory. The dark web, unsanctioned marketplaces, and social media groups promise fast delivery, but these deals rarely end well. Unregulated chemicals bring unknown risks, legal trouble, and danger to health and safety. Laws target both shady suppliers and unwary buyers.
For newcomers facing these hurdles, it helps to consult established researchers, procurement officers, or compliance experts. Often, universities and research institutions will already have supplier contracts, shipping solutions, and skilled staff familiar with import regulations and customs clearance. It takes teamwork to break down the barriers of tight regulation.
If legal and ethical lines remain clear, and research stands on solid ground, there’s always a solution available through professional channels. Taking shortcuts or guessing at supplier reputability leads to more problems than solutions. Over time, patience and the right support network make the difference between long-term scientific work and costly missteps.
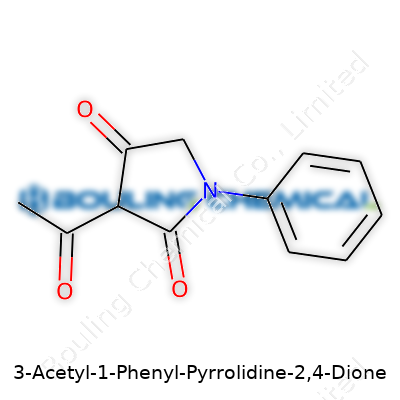
| Names | |
| Preferred IUPAC name | 1-Phenyl-3-acetylpyrrolidine-2,4-dione |
| Other names |
3-Acetyl-1-phenylpyrrolidine-2,4-dione 3-Acetyl-1-phenyl-2,4-pyrrolidinedione 2,4-Pyrrolidinedione, 3-acetyl-1-phenyl- 3-Acetyl-1-phenyl-2,4-dioxopyrrolidine Phenylacetylsuccinimide |
| Pronunciation | /ˈθriː əˈsiːtɪl wʌn ˈfiːnɪl paɪˈrɒlɪdiːn ˌtuː fɔːr ˈdaɪoʊn/ |
| Identifiers | |
| CAS Number | 60118-89-8 |
| 3D model (JSmol) | `3Dmol='C1(CC(=O)NC(=O)C1)C2=CC=CC=C2'` |
| Beilstein Reference | 120873 |
| ChEBI | CHEBI:91191 |
| ChEMBL | CHEMBL137763 |
| ChemSpider | 20798836 |
| DrugBank | DB07715 |
| ECHA InfoCard | 19c2a58f-5bb1-4bd5-90e7-9041e553dab6 |
| EC Number | 3.5.2.16 |
| Gmelin Reference | 79417 |
| KEGG | C18752 |
| MeSH | D000198 |
| PubChem CID | 21416443 |
| RTECS number | UG4375000 |
| UNII | 8B6Q9SN54M |
| UN number | Not regulated |
| Properties | |
| Chemical formula | C12H11NO3 |
| Molar mass | 233.23 g/mol |
| Appearance | White to pale yellow solid |
| Odor | sweet, floral |
| Density | 1.24 g/cm³ |
| Solubility in water | slightly soluble |
| log P | 0.2 |
| Vapor pressure | 0.0000221 mmHg at 25°C |
| Acidity (pKa) | 8.7 |
| Basicity (pKb) | pKb = 13.42 |
| Magnetic susceptibility (χ) | -70.29 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.554 |
| Viscosity | Viscosity: 1.09 cP (25°C) |
| Dipole moment | 4.89 Debye |
| Thermochemistry | |
| Std enthalpy of formation (ΔfH⦵298) | -424.1 kJ/mol |
| Pharmacology | |
| ATC code | N02BG06 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P272, P302+P352, P321, P362+P364, P501 |
| Flash point | 115.3 °C |
| Lethal dose or concentration | LD50 oral rat 640 mg/kg |
| LD50 (median dose) | LD50 (median dose): **320 mg/kg (Rat, Oral)** |
| NIOSH | LM2975000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 3-Acetyl-1-Phenyl-Pyrrolidine-2,4-Dione is not specifically established by OSHA. |
| REL (Recommended) | 10 mg/m3 |
| Related compounds | |
| Related compounds |
Barbituric acid Phenylbutazone Pyrrolidine Acetylacetone Succinimide |