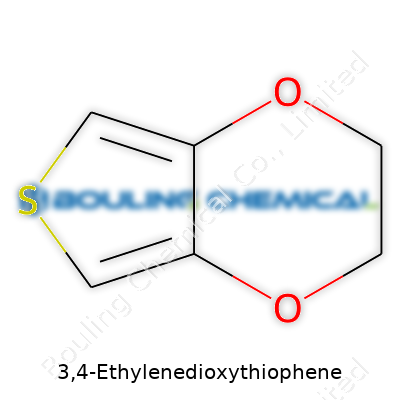
Back in the late 20th century, researchers searching for new organic materials with conductivity potential stumbled across 3,4-ethylenedioxythiophene. The story of EDOT grew out of the hunt for better polymers, especially ones capable of moving electricity with some flexibility and reliability. Early studies opened the door for the production of its polymer form, PEDOT, catching the eye of scientific communities dealing with electronics, energy, and even biology. Over the decades, efforts from university labs and industrial groups pushed EDOT from academic journals to products you can spot in real-world tech. Once prized for academic curiosity, it now sits in the supply chain of smart devices, touch screens, even solar panels.
Buyers typically find 3,4-ethylenedioxythiophene as a colorless to pale yellow liquid, often stored in tightly sealed containers due to its sensitivity to airborne moisture and light. Industrial producers, such as Heraeus and Sigma-Aldrich, offer EDOT as a research-grade monomer, as well as in bulk for large-scale polymer manufacture. Most distributors slap CAS Number 126213-50-1 on the label. End users might see names like EDOT, or (2,3-dihydrothieno[3,4-b][1,4]dioxin) if peeking at detailed technical data sheets. No matter the source, quality concerns often circle around purity and moisture content – anything less than 98% pure can spoil precise polymerizations.
What’s remarkable about EDOT is how this little aromatic molecule packs a punch. The liquid has a boiling point just above 200°C, resists dissolving in water, but blends well with organic solvents such as chloroform or acetone. The smell—slightly ether-like—signals its reactivity, so labs rarely leave it sitting out. Electron-rich sites in the molecule make it eager to polymerize with oxidative agents, transforming into dark, shiny films in minutes. While its liquid form seems unassuming, the real magic appears after polymerization, when it turns into a solid that conducts electricity and fends off oxidation better than many rivals.
Barcode scanners and inventory logs in chemical warehouses often point to EDOT with product codes like CSEL-093010 or 409447. Datasheets list its molecular weight at 142.18 g/mol and molecular formula as C6H6O2S. Typical products arrive with purity levels above 98%, and suppliers provide info on UV-vis absorption, melting point (usually below room temp), and moisture content. Containers bear warning labels relating to toxicity and humidity. Storage instructions stress a cool, dry spot, away from acids and bases. Shelf life can get cut short by sunlight, so good stockrooms keep the bottles in amber vials or foil-wrapped containers.
Synthesis in industry often relies on the alkylation of thiophene, feeding diols and oxidizing agents under controlled temperatures. Yields depend upon careful temperature control and removal of byproducts. Researchers working bench-top scale can prepare EDOT by reacting 3,4-dihydroxythiophene with ethylene glycol derivatives in the presence of acid catalysts. Separation often involves vacuum distillation, as impurities can hinder later polymer growth. The difference in color and odor often hints whether the batch will lead to high-performance plastics or head to the waste bin.
EDOT embraces modification, especially through electropolymerization and oxidative chemical polymerization. Its most celebrated reaction uses oxidizers such as FeCl3, transforming the monomer into conductive PEDOT. Chemists tweak the substituents on the ethylenedioxy ring, adjusting properties like solubility and light absorption. Other reactions explore cross-linking, adding functional groups for bio-compatibility or energy storage. Its ease of polymerization lends itself to fine-tuning, letting scientists grow films of custom thickness for any sort of device.
Scan catalogs and you’ll see synonyms: 3,4-ethylenedioxythiophene, EDOT, 2,3-dihydrothieno[3,4-b][1,4]dioxin. Once in product blends, companies brand the polymerized form under catchy short names like PEDOT or Clevios. Confusing things a step further, regional distributors occasionally add their stock numbers or translation-based names. For sourcing or patent searches, sticking to CAS 126213-50-1 tends to cut through the tangle of names and translations.
Handling EDOT is no casual affair—lab workers wear gloves, goggles, and sometimes respirators due to vapor concerns. The liquid can irritate skin, eyes, and respiratory tracts. Proper ventilation keeps accidental exposure in check, and best practice means keeping spill kits handy. Material Safety Data Sheets spell out incompatibility with oxidizing agents and open flames. Storage regulations mirror those for other volatile organics: cool and dry, with strong labeling. Firms in the EU or US have to keep records, as REACH and OSHA rules apply, including annual review of storage protocols.
Polymerized EDOT gets a front-row seat where lightweight, flexible conductors matter. You’ll find it layered in touch screen coatings, organic solar cells, antistatic films, and flexible printed circuitry. Its low band-gap makes PEDOT a favorite for high-definition displays and high-speed capacitors. Medical researchers see value in its biocompatibility, using it in sensors and nerve interface devices. Manufacturers that build organic light-emitting diodes (OLEDs) choose EDOT-based polymers for transparency and endurance. In my own university lab days, PEDOT films popped up on everything from test sensors to classroom demonstrations about emerging “plastic electronics.”
Work never seems to slow. Teams worldwide experiment with EDOT copolymers, chasing better durability, environmental stability, or biointerface performance. Newer methods of electropolymerization—using green solvents or lower temperatures—surface in journals every month. The chase to replace rare or toxic elements in batteries has researchers designing EDOT derivatives that store and shuttle ions more efficiently. At industry expos, startups demo inks and coatings promising faster wireless charging or thinner smart wearables, all possible because of tweaks to the way EDOT chains connect and grow.
Toxicity stands as a real concern for workplace safety and product lifecycle. Animal studies suggest moderate toxicity: inhalation or skin contact causes irritation and, in larger doses, nervous system effects. Disposal requires incineration or careful chemical deactivation, given the risk to aquatic systems. Polymerized forms, such as PEDOT, shed less concern, but regulatory bodies still monitor production waste and end-of-life disposal. Ongoing long-term studies seek to clarify effects in medical settings, especially where PEDOT might contact tissue, blood, or organs over years of device operation.
Trends in wearable tech, smart textiles, printed batteries, and flexible solar panels point to broader use of EDOT-based polymers in coming years. Ongoing improvements to preparation methods, using renewable feedstocks or cleaner catalysts, look poised to reduce environmental impacts. Greater integration into medical devices hinges on clearing toxicity concerns, but small-scale studies seem promising for applications like biosensors and nerve guidance scaffolds. If my own experience working on flexible electronics says anything, it’s that demand for customizable, robust conductors is growing, not shrinking. Most forecasts suggest that EDOT and its chemical cousins won’t just stay relevant—they’ll become common as copper used to be in the last century’s technological leap.
3,4-Ethylenedioxythiophene, or EDOT, tends to show up where we often least expect it: inside screens, sensors, and devices most people don’t even think twice about. Years ago, my phone’s touchscreen dragged and missed taps, even though it was new. I learned the ‘invisible’ parts of technology make all the difference—EDOT is a pretty good example.
The real magic behind EDOT comes out when scientists turn it into a polymer called PEDOT. Unlike copper and other bulky conductors, PEDOT lets engineers create thin, flexible films. These films end up in smartphones, tablets, and even solar cells, carrying electricity across surfaces that bend, twist, and stretch. Try flexing a copper wire over and over—it snaps. PEDOT holds up.
I remember soldering wires for a project in college. The dream was to build a super-light sensor that measured weather right through the window, no ugly wires winding all over. Conductive polymers made it possible, letting sensors work on thin plastic without the weight or hassle.
Walk into any hospital and you’ll find sensors everywhere—heart monitors, brain caps, wearable patches. Traditional metal leads irritate the skin and break down with sweat. PEDOT’s superpower is its friendly interface with both electronics and the human body. Scientists coat electrodes with PEDOT, making long-wear devices possible, even for folks with delicate or sensitive skin.
I once had a family member wear a cardiac patch. A week later, there wasn't the usual red, itchy spot you might expect. Devices like those often use conductive polymers at their interface, keeping discomfort down for people who already have enough to worry about.
Solar panels used to be heavy and rigid. PEDOT offers a workaround. Thin, transparent layers coat flexible panels that roll out on rooftops, windows, or backpacks. These films let sunlight pass while transmitting electric signals underneath. Just imagine rolling up a solar panel, tossing it in your bag, and charging gadgets with sunlight anywhere you go.
Researchers have reported PEDOT’s use in organic solar cells—lighter, more adaptable, and, in some cases, cheaper than old-fashioned silicon panels. The trade-off can be efficiency or lifespan, but new blends and tweaks to the EDOT structure are closing the gap every year.
Smart clothing wants fabric that senses, stretches, and conducts like wire without turning stiff. Here, PEDOT shines again. Yarn dipped or spun with PEDOT polymer becomes part of a shirt that tracks heart rate, movement, or even the local air quality.
Little by little, EDOT is weaving into the fabrics of daily life—sometimes literally. It gives designers and engineers a chance to build things around people, not just plug people into things.
While a lot about EDOT sounds like science fiction, it comes with its own headaches: handling safety in labs, controlling byproducts, and finding ways to make this tech affordable enough for everyone. Companies and universities are digging into greener chemical processes, safer recycling, and new recipes for even better results.
If you’ve ever wondered what turns science ideas into stuff you actually use, EDOT is a good reminder that the right chemistry can make technology friendlier, lighter, and more a part of regular life.
Every chemist knows what it’s like to worry about a leaky bottle or a rogue humidity spike. Storing chemicals brings its own set of headaches. Take 3,4-Ethylenedioxythiophene. The name might sound complicated, yet handling this liquid isn’t much different from other common lab solvents, aside from a few specific quirks. People call it EDOT for short, and it’s become popular among researchers creating conductive polymers, especially PEDOT. EDOT loves to polymerize, but only in the right conditions, so without some care, you end up with a useless mess instead of a precious reagent.
I remember the first time our lab group ordered EDOT. The bottle came snug in a foil wrap, with this warning in fat red letters: “Protect from light and heat.” The moment light or random heat gets involved, the risk of premature polymerization goes up fast. I once left a bottle too close to a sun-lit window; within days, the contents thickened, color deepening from water-clear to a murky brown. That small slip-up wasted both time and money. The lesson stuck. After that, I always put every fresh batch in a refrigerator, keeping it away from fluorescents and direct sunlight. The rule I follow: store it at 2-8°C. It may cost a few extra minutes for things to warm up to room temp before use, but the bottle stays fresh for months instead of weeks.
Moisture and EDOT don’t mix. Even though EDOT sits in the liquid state, a single drop of water encourages side reactions and unwanted gunk. I once watched a careless coworker pipette EDOT with a damp tip—what emerged a week later was cloudy and thick, doomed for the disposal container. Keeping water out means sealing the container tightly and never leaving the cap off longer than needed. A dry atmosphere, or even a desiccator, makes a huge difference for long-term storage, particularly in humid climates. For jobs involving big batches or industry-scale setups, nitrogen blanketing comes into play, but even at the bench, a basic desiccant pack inside the storage cabinet offers peace of mind.
Glass trumps plastic for storing EDOT. The compound eats away at some plastics over time, so a good amber glass bottle paired with a Teflon-lined cap wins every time. I keep a small quantity in a working vial to reduce exposure to the main stash. Labeling dates and keeping an eye on color shifts help catch trouble before it ruins a reaction. I hear stories of people using parafilm as a seal, but it pales in comparison to a dedicated screw cap.
Building good habits around chemicals like EDOT saves cash and time, and keeps experiments safe. Following these straightforward steps—cool storage, light protection, dry handling, and solid containers—makes a difference. People sometimes cut corners, thinking a few days won’t matter. Trust me, if you value clean results and less stress, stick with routines that protect your reagents. A good bottle of EDOT can carry a semester’s worth of work; all it takes is a little effort to keep it that way.
You might not hear about 3,4-Ethylenedioxythiophene (often called EDOT) at a family dinner, but it pops up where new tech grows fast. Makers use it for things like solar panels and antistatic coatings. The question many folks have is: does EDOT put lives, water, or air at risk?
Researchers have tested EDOT and found it doesn’t act like many heavy metals or old-school solvents that easily poison land and lungs. It doesn’t evaporate quickly, and it’s not known to float around in dust the same way as things like lead. A study from BASF flagged EDOT as “moderately” toxic to aquatic life, meaning if a whole bunch of it got into rivers, fish would start to feel the hit. But the risk to everyday city folks, or even factory workers with halfway decent protective routines, stays lower.
I once spent time helping a friend in an electronics lab. Gloves, lab coats, and good ventilation kept us from breathing fumes or soaking chemicals through our skin. The fact is, EDOT vapor isn’t everywhere–not like gasoline at a busy pump. If labs and small factories keep spills off the ground, check air vents, and store it tight, risk falls even lower. In these places, accidents tend to happen because someone skips steps, not because a substance leaps out of a bottle all on its own.
Households rarely face EDOT directly. It doesn’t hide in cleaning sprays or hardware store aisles. Its biggest environmental worry shows up if a production spill hits a riverbed. Tests on daphnia (tiny crustaceans that feed fish) showed harm at fairly low amounts, so runoff matters. Across the world, several groups track chemical discharges from factories; when EDOT runs loose in water, it needs quick attention because streams don’t clean it up overnight.
I’ve chatted with people in the plastics and coatings industry. Some complain about dry skin or headaches, but no big waves of health crises linked directly to EDOT have made headlines. Skin contact, especially if it goes on for days, can bring rashes or allergies–the kind that clears up if you cut contact. One report from Europe pointed out possible mild irritation in eyes or lungs if it’s mishandled as dust or a hot vapor. Respirators, goggles, and gloves block most of these issues.
Problems start small—spills in a factory, leaks down a drain. Workers and managers actually stop trouble before it spreads by watching the flow from containers and using spill kits. Regular waste checks and filters on drains cut pollution sharply. In my own work, clear labels and cheat sheets for new staff proved more valuable than any regulatory paperwork could ever track. Simple habits, like keeping storage capped and cleaning benches at the end of each shift, build safety into everyday routines.
No chemical comes with zero risk. That said, EDOT can often look scarier than it turns out to be, especially if handled with basics in place. We live in a world built with thousands of chemicals, and it comes down to choices: treat the bottles with focus, plug the drains, shield your skin, and tell the next person how to do the same. Respect, rather than fear, builds a safer space for science and society.
Staring at a small vial of 3,4-ethylenedioxythiophene (or EDOT, if you love chemistry shorthand) doesn't exactly shout technology revolution. Yet that is exactly what goes on in university labs, tech startups, even high school competitions these days. Polymerizing EDOT turns it into PEDOT—a mouthful, yes, but also a workhorse behind some of today's flexible screens and neural implants. I remember the surprise on my face the first time I saw pure, clear EDOT morph into a dark blue, electrically conducting film. The magic is pure chemistry but the impact hits a lot of real-world problems.
Getting from monomer to conducting polymer starts with simplicity. Take EDOT and give it the right oxidant. Ferric chloride (FeCl3) works well. Pour a solution of EDOT over a substrate—something like glass or a PET film used for phone screens. Then add a solution of FeCl3 and wait for the blue-black PEDOT film to form. Temperature matters, sure, but I’ve seen good films made in open air, with little more than a beaker and some patience.
There’s an alternative that relies on electrical current. Here, electrodes get dipped in an EDOT solution while a mild voltage runs between them. Electropolymerization needs a power supply but rewards your effort with thin, even films. It’s almost art—paint with electrons instead of a brush. I tried this approach in a demo for high schoolers, and the look of wonder they had said it all: this is chemistry you can see, touch, and use.
PEDOT isn’t just science for science’s sake. When you coat a surface with PEDOT, you build a film that carries electricity, flexes, and resists water. I ran across it first browsing research in solar cells, but the medical side keeps growing. Brain electrodes with PEDOT coatings record signals more cleanly. Hearing aids last longer. Sensors fit onto skin or fabric. Add some polystyrene sulfonate (PSS) during your reaction and you get PEDOT:PSS, which spreads in water—a trick that turned lab experiments into printed electronics.
Many students, even outside chemistry, end up working with PEDOT for class projects or early careers. That hands-on path—from monomer vial to working device—teaches more about the connections between fields than textbooks ever will. I once mentored a student team working on smart clothing. They faced issues with film peeling and uneven conductivity. Swapping solvents, tweaking EDOT to oxidant ratios, or using better rinses all helped, but direct feedback—film color, electrical measurements—guided each step.
Working with EDOT presents challenges. Oxidants like FeCl3 stain and corrode; waste disposal isn’t as simple as pouring down the drain. Cheaper, greener oxidants and processes would widen access, especially for classrooms working on tight budgets. Scaling up brings new hurdles: thicker films crack, thin ones struggle with stable conductivity. My experience found simple fixes—lowering oxidant concentration, tweaking drying times, better substrate cleaning—often did more than expensive equipment upgrades.
One promising idea: community-driven protocols that adapt recipes for local resources. Students in one workshop blended EDOT polymerization with natural stabilizers from plant extracts, making films for bio-sensing without expensive chemicals. Their results weren’t perfect, but the direction signals a future where lab magic leaves the bench. Polymerizing EDOT connects disciplines, markets, and cultures; it’s a piece of storytelling written in electric blue.
Folks who work in labs or mess around with polymer science tend to get used to long chemical names. 3,4-Ethylenedioxythiophene (EDOT) is one such compound that pops up a lot these days, especially when digging into the world of modern electronics and biosensors. Its molecular weight clocks in at around 142.18 grams per mole. Plenty of calculators and textbooks spit out this number, but knowing what it means—and why it matters—calls for more than typing some numbers into a spreadsheet.
Molecular weight might seem dull on the surface, like a detail you’d only care about for exam points. But anyone who’s ever had to plan a reaction or scale something up for production can tell you otherwise. I’ve watched a colleague waste entire days—maybe even weeks—mixing up batch sizes because they forgot to check the molecular weights in their protocol. That one slip turned into ruined experiments, lost money, and a pretty grumpy team.
For EDOT in particular, this number tells you how much of it you’re actually handling. Since this stuff often gets converted into PEDOT, one of the go-to materials for transparent electrodes and flexible electronics, even a small error can spiral. In our workplace, that’s meant ordering too much solvent or coming up short when we reached the test phase. Multiply that by a manufacturing run, and costs add up fast.
This isn’t just about budgeting, either. Take electrochemical polymerization, where the right ratio is everything. A mistake in the molecular weight calculation can throw off conductivity, color, or even the physical structure of what you’re trying to make. I’ve seen folks toss out entire batches that didn’t pass as “conductive enough.” Those kinds of mistakes don’t sit well when money’s tight or supply chains are still shaky.
Molecular weights also sneak into environmental rules. Safety officers and compliance folks track every gram moving through the facility, especially since these reagents need careful handling. If you estimate wrong, waste streams no longer line up with regulations. I’ve heard stories—sometimes horror stories—of labs hit with surprise audits because their inventory didn’t match waste disposal paperwork.
Some labs lean into tech to sidestep these headaches. Barcode readers, smart scales, and inventory software can cut down on human error. Even small research teams can sync up their protocols and numbers, using shared digital tools so nobody operates in the dark. Our team started doing this after a couple of embarrassing misses and the process is smooth now.
Training doesn’t get enough credit, either. For students or new staff, learning to double-check calculations can make or break a project. I make a habit of running through a sample calculation with every new hire, because seeing the steps makes the impact of a miscalculation real. Some people think of science as luck or genius, but most of the wins I’ve seen come from careful, boring prep—and molecular weight calculations fit right in.
All told, that 142.18 grams per mole figure may look plain, but its consequences reach into every corner of applied science. Getting it right gives you a shot at inventing something that actually works outside the lab, in the real world. And in my book, that’s what separates a random experiment from real progress.
| Names | |
| Preferred IUPAC name | 2,3-dihydrothieno[3,4-b][1,4]dioxine |
| Other names |
EDOT 3,4-Ethylendioxythiophen 3,4-Ethylenedioxy-thiophene 3,4-(1,2-Ethanediyloxy)thiophene |
| Pronunciation | /ˈɛθ.ə.lɪn.iˌdaɪ.ˈɒk.si.θaɪ.ˌɒ.fin/ |
| Identifiers | |
| CAS Number | '126213-50-1' |
| Beilstein Reference | 120857 |
| ChEBI | CHEBI:52540 |
| ChEMBL | CHEMBL1231373 |
| ChemSpider | 14131 |
| DrugBank | DB14116 |
| ECHA InfoCard | 12b9a2a2-f1ed-42b7-954c-65e5c2d3dcbb |
| EC Number | 'EC 607-797-3' |
| Gmelin Reference | 78746 |
| KEGG | C11825 |
| MeSH | D000072633 |
| PubChem CID | 119176 |
| RTECS number | KH8580000 |
| UNII | N31VJE63ZB |
| UN number | UN3434 |
| CompTox Dashboard (EPA) | DV9772X6O2 |
| Properties | |
| Chemical formula | C6H6O2S |
| Molar mass | 142.18 g/mol |
| Appearance | colorless to light yellow liquid |
| Odor | Odorless |
| Density | 1.34 g/mL at 25 °C |
| Solubility in water | slightly soluble |
| log P | 0.44 |
| Vapor pressure | 0.177 mmHg (25 °C) |
| Acidity (pKa) | 12.07 |
| Basicity (pKb) | pKb = 12.86 |
| Magnetic susceptibility (χ) | -35.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.520 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.17 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 156.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -107 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1421 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | Health: 2, Flammability: 2, Instability: 1, Special: |
| Flash point | 113 °C |
| Autoignition temperature | 400°C |
| LD50 (median dose) | LD50 (median dose): >2000 mg/kg (rat, oral) |
| NIOSH | KWZ36 |
| PEL (Permissible) | Not established |
| Related compounds | |
| Related compounds |
Thiophene Furan Pyrrole 3,4-Ethylenedioxythiophene polystyrene sulfonate (PEDOT:PSS) 3,4-Propylenedioxythiophene |