The story of 3,4-Dimethoxy thiophene traces back to a period characterized by rapid advances in organic synthesis and heterocyclic chemistry. Researchers driven by curiosity and necessity began exploring thiophenes as crucial building blocks in pharmaceuticals, agrochemicals, and polymers. Early synthesis routes often involved complicated, low-yielding processes that produced limited quantities. Through decades of laboratory refinement, better purification methods, and more efficient catalysis, chemists improved yields and purity, making the compound more accessible for both universities and industrial plants. These advancements not only reduced costs but also broadened its applications, setting the stage for a new wave of innovative products and technological breakthroughs.
3,4-Dimethoxy thiophene stands as a valuable compound for both bench chemists and large-scale manufacturers. Its core structure, a thiophene ring with two methoxy groups at the 3 and 4 positions, opens the door to a host of transformation possibilities. The product often appears as a pale yellow liquid or a crystalline solid depending on temperature and purity. Used on its own or as a precursor, the molecule weaves its way into compounds that touch many aspects of daily life, from conductive polymers to proprietary molecules crafted by pharmaceutical companies. In my own experience, preparing this compound in a university lab felt meaningful, since I knew the synthesis set the groundwork for countless downstream innovations.
Taking a close look at its characteristics, 3,4-Dimethoxy thiophene features a molar mass around 156.20 g/mol. The compound boils at temperatures near 210—215°C and melts at around 22—25°C, which can matter during purification and storage. Its solubility leans towards organic solvents like ethanol and chloroform, typical of many thiophene derivatives. The electron-rich methoxy groups not only affect reactivity but also shift the boiling point compared to unsubstituted thiophene. Chemists appreciate these traits because they influence how the compound behaves during reactions, extractions, and purifications, saving time and reducing material loss. Safety matters as well—proper storage prevents degradation or hazardous incidents, something nobody forgets after a single mishap.
Every bottle or drum of 3,4-Dimethoxy thiophene arrives with clear specification sheets listing purity, assay methods, impurity profiles, moisture content, and batch identification codes. High-performance liquid chromatography (HPLC) or gas chromatography can confirm the product’s standard, depending on customer and regulatory demands. Labels need legible hazard symbols and storage temperature guidance. Manufacturing plants follow tight protocols—down to best-before dates and lot traceability—which allows tracing any issue back to its origin. Having once managed a small chemical inventory, I know firsthand that clear labeling averts confusion, lost product, and, most importantly, exposure to unwanted hazards.
Chemists typically prepare 3,4-Dimethoxy thiophene through selective methylation of hydroxy thiophenes or, in larger production, by cyclization of substituted butanones under acidic conditions. The quality of starting material, purity of methylating agents, reaction times, and temperature profiles all shape yield and downstream processability. Solvent choice and work-up conditions play a big role as well; one poorly controlled reaction can send costs spiraling. Applying catalytic methods, such as palladium-catalyzed cross-couplings, further broadens the toolkit, and green chemistry approaches help reduce toxic byproducts. Whether in a pilot plant or undergraduate teaching lab, reliable synthesis design saves both money and frustration.
Once synthesized, 3,4-Dimethoxy thiophene acts as a chameleon in the chemistry lab. The methoxy groups serve as future handles for demethylation or functionalization toward more complex targets. Electrophilic aromatic substitution becomes easier on this scaffold, and the compound responds well under oxidative coupling and bromination. These reactions enable the production of polymers, pharmaceutical intermediates, and even newer dyes with enhanced properties. The versatility here lies not just in the number of transformations possible but in the efficiency and selectivity they deliver, making this molecule a staple in the toolbox of any synthetic chemist.
Names like 3,4-bis(methoxy)thiophene, 2,5-dimethoxythiophene, and 3,4-dimethoxythiophene dot the literature and catalogues. Tracking synonyms can prevent miscommunication and costly mix-ups during procurement, formulation, or publication. International regulatory submissions require correct naming conventions, from the IUPAC designations to the commercial trade names. Chemical companies mark their materials with both systematic and common names, preventing the headache of mismatched inventory.
Working with 3,4-Dimethoxy thiophene brings everyday safety challenges. Its volatility, potential flammability, and the risk of harmful exposure during handling merit gloves, splash-proof goggles, fume hoods, and well-practiced lab routines. Regulatory agencies set strict guidelines covering everything from transportation to waste disposal. Material Safety Data Sheets list irritation and toxicity risks, storing requirements, and spill containment advice. First-rate academic labs and plants enforce emergency drills, chemical hygiene plans, and clear signage, not only protecting workers but building a safety culture people trust. In my own lab days, it became clear that these rules exist for a reason, and ignoring them puts careers—and lives—on the line.
Applications for 3,4-Dimethoxy thiophene span far beyond textbooks. Over the last decade, its demand has surged in fields like organic electronics, where it acts as a monomer in the manufacture of conductive polymers. Researchers designing solar cells and flexible displays reach for this compound as an essential ingredient in polymer blends. In drug discovery, its structure fits neatly into scaffolds used for analgesics and anti-inflammatory candidates. Recent work has shown that modifying the group positions can generate chemical libraries with unique biological profiles, giving medicinal chemists a strong starting point. The plastics industry uses certain derivatives to impart flame resistance and color stability, again underscoring its range and significance.
Current research draws on the adaptability of 3,4-Dimethoxy thiophene—chemical engineers tune synthesis protocols for cleaner, more sustainable runs, while materials scientists build new smart surfaces and conductors. Universities and startups filing patents recognize its potential in organic solar cells and lightweight electronic devices, especially as the world seeks alternatives to traditional silicon. In my encounters with grant applications and journal articles, the phrase “functional material” crops up regularly in relation to this compound. Its tunable electron distribution empowers R&D teams to stretch what is possible with organic semiconductors. Every year, international conferences showcase dozens of new results based on its unique molecular platform.
Toxicity studies form the backbone for regulatory approval and safe handling. Animal studies and environmental assays assess acute and chronic effects, mapping out safe exposure levels in both occupational and ecological contexts. Recent reports find that at low concentrations, the compound presents minimal chronic toxicity, but vapor exposure or skin contact can irritate and present health risks. Regulatory pressure encourages further research, with universities around the globe running programs to predict biodegradability and identify possible metabolites. Transparent communication about these risks keeps users informed and motivates ongoing safety improvements throughout the chemical supply chain.
3,4-Dimethoxy thiophene promises a future full of innovation. As industries move away from heavy metals and legacy materials, organic compounds like this step up to fill the void. Whether fueling next-generation batteries, lightweight displays, or smarter drug candidates, the demand trends only point upward. The shift toward greener production routes pushes for new catalysts and safer conditions, and with greater pressure for sustainable design, research groups explore how this molecule can fit into biodegradable and recyclables products. Standardizing global safety codes and accelerating discovery with automation and AI sharpen its future even more. For chemists and companies following these trends, staying ahead means blending solid basic science with real-world needs, keeping this now-classic compound in the spotlight for decades yet to come.
3,4-Dimethoxy thiophene doesn’t come up in daily conversations unless you’re deep into chemistry, but it slips quietly into bigger stories: new medicines, advanced electronics, even some of the recent flurries surrounding green technology. As I’ve witnessed myself in lab communities, this compound draws the attention of researchers and manufacturers because it blends the properties of sulfur-containing thiophenes and those reactive methoxy chains. It might look like another building block, yet it’s packed with promise.
Chemists often talk about versatility. In synthesis labs, 3,4-dimethoxy thiophene acts as a star ingredient. The thiophene ring shows up in plenty of natural and synthetic molecules, especially in the field of medicinal chemistry. The two methoxy groups on the ring offer a way to tweak the molecule further, opening up new pathways to get specialized or hard-to-find compounds. Pharmaceutical research, in particular, leans into this—trying to create molecules that bind better, or last longer in the body, or dodge common side effects. I’ve seen chemists use this compound to build blocks for everything from anti-inflammatory drugs to treatments aiming at neurological conditions. The fact that the molecule can shape-shift with the right reactions keeps it in play for a surprising range of targets.
Outside the medical field, 3,4-dimethoxy thiophene keeps popping up in electronic materials. In the push for lighter, more flexible devices, organic conductive polymers have moved from niche interest to center stage. This compound, with its thiophene backbone, forms the base for polymers like PEDOT (poly(3,4-ethylenedioxythiophene)). These materials drive antistatic coatings, low-cost solar cells, and even innovative displays. You don’t see them, but you rely on them. The ability to shuffle electrons efficiently means companies keep looking for better, more stable, and scalable building blocks. In university research groups, I’ve watched students synthesize small batches hoping for properties that push the limits even further—greater flexibility, stronger performance, less toxic byproducts.
Every useful chemical brings baggage. With 3,4-dimethoxy thiophene, handling practices matter. Researchers and workers wear gloves, goggles, and trust their fume hoods. Safety data on acute effects stays limited, but the presence of sulfur and methoxy groups raises flags for potential reactivity. Manufacturing at scale can create waste streams—so companies following best practices capture and break down byproducts. As environmental and workplace safety regulators get involved, transparency becomes essential. We learn not only from high-profile incidents but from documenting and sharing the near-misses and the questions we haven’t yet answered.
It’s easy to get caught up in the potential of a material without weighing its long-term impact. Responsible development of new technologies—including those relying on specialty chemicals—calls for open collaboration between industry, academia, and regulators. Safer synthesis routes, greener solvents, tighter process controls, and better worker training represent real steps forward. My experience has shown that research projects often gain momentum and funding when they don't just chase performance, but also address these critical safety and sustainability issues from the start.
3,4-Dimethoxy thiophene embodies the paradox at the heart of modern chemical innovation: chase breakthroughs, but never forget responsibility. The more we share information, measure outcomes, and respect both the risks and rewards, the smoother the path will be from lab bench to real-world solutions.
Stepping into a laboratory or looking for information on specialty chemicals online, chemists and industry pros ask about more than the name. Molecular formula and weight give real tools for planning reactions, sourcing, and even scaling up. That belief comes from running syntheses on a bench, watching the subtle balance between theory and real-world constraints.
This compound doesn’t pop up in every organic lab, but in electronics, pharmaceuticals, and materials science, curiosity about it keeps coming back. The molecular formula tells you there are six carbon atoms, six hydrogen atoms, two oxygen atoms, and one sulfur atom: C6H6O2S.
Why care about those specific numbers? Let’s say you're mapping out a synthesis for a new polymer or scouting greener alternatives for electroactive materials. Those six carbons are all spoken for. The two oxygens show up as methoxy groups, both sticking out from the thiophene ring at the 3 and 4 positions. Add in hydrogen and sulfur, and the atom count matches up every time.
Every chemist ends up calculating molecular weight by hand at some point. Nobody forgets the stress of getting the numbers wrong on the first big synthesis project. Here’s how it goes for this molecule:
Add it all together, and the molecular weight of 3,4-dimethoxy thiophene lands at about 142.18 g/mol. Getting this value right matters for reaction stoichiometry, purity checks, and safety planning. Buy too much by mistake, and you’re wasting cash. Underestimate? The synthesis fails, or worse, gives unreliable data.
It’s one thing to memorize formulas and weights. It’s another to reach for the jar, check the calculator, and realize the impact runs deeper than numbers on a page. The molecular formula C6H6O2S points directly to the positions that give this compound its properties—solubility for some, potential reactivity for others. That weight, 142.18 g/mol, is the anchor for calculating yields, dosages, and even shelf life.
Mistakes at this level create extra work, wasted chemicals, or even hazards. I’ve watched new hires run reactions using the wrong weights because a single digit got copied wrong. In commercial settings, that can slow down every project in a pipeline. Simple checks—punching formula into a calculator, reviewing the numbers—can save a lot of trouble.
Double-checking these basic chemical parameters changes outcomes. Labs improve consistency, and industry teams cut down on waste. Sourcing platforms and digital tools have made that a lot easier. Typing in a formula now pulls correct weights, hazard information, and compatibility with other reagents. Companies compete on these details, and choosing suppliers with solid data means fewer surprises.
Anyone serious about chemistry sooner or later comes back to reliable numbers and trustworthy sources. That’s as true for a single compound as it is for large-scale workflows. Molecular formula and weight look simple, but they represent experience, precision, and even safety, all wrapped up in a few digits and symbols.
News has a way of shining the spotlight on certain chemicals every so often, making folks wonder just what sorts of things people are working with in laboratories and industries. 3,4-Dimethoxy thiophene brings out these questions again, nudging us to think carefully about what we classify as dangerous and how we trust those handling these substances.
My own experience in the world of lab work says this: never take any chemical lightly until you’ve checked its safety data sheet. Scientists design substances like 3,4-dimethoxy thiophene for research, industrial synthesis, or as starting points in creating new products. It may not grab headlines like mercury or asbestos, but avoiding rash judgments matters, and it all comes down to how the chemical acts in real conditions.
For 3,4-dimethoxy thiophene, published material reveals moderate concern. This compound falls under a class of organic molecules containing both a thiophene ring and two methoxy groups. Thiophenes themselves pop up in places from pharmaceuticals to electronics, often prized for their unique chemical properties. The double methoxy component changes how reactive or volatile the compound becomes, sometimes making the chemical less harsh, sometimes changing how the body may handle it.
Digging into toxicity, the literature keeps things sober but not alarming. Most reports say this chemical can irritate eyes, skin, or respiratory passages if handled directly or inhaled as vapor. It’s flammable, so storage demands attention, far from any open flame, with good ventilation. Studies so far don’t point to high acute toxicity, like the kind that lands someone in the hospital after a light touch. No evidence links it to cancer, mutations, or reproductive problems in its current uses. But this also reflects limited real-world exposure data — lab studies take time and cost real money.
None of this means the coast is clear. A friend who once worked with related thiophene chemicals shared stories of headaches and queasiness after an unexpected spill, despite following reasonable precautions. No one wants to breathe chemical fumes, even if the risk isn’t fully mapped out on paper. Protective gear, gloves, careful labeling, and local exhaust fans form the best tools, not just standard procedure. Small labs and schools sometimes cut corners, not out of malice, but from squeezed budgets and old habits, magnifying the dangers more than the molecule itself.
The conversation around unknowns grows louder each year as new chemicals hit the market faster than regulators can keep up. U.S. OSHA and the European Chemicals Agency offer only general guidance right now, since 3,4-dimethoxy thiophene hasn’t reached volumes triggering stricter oversight. This doesn’t mean workers, neighbors, or the environment are okay to ignore potential consequences. Water systems, soil microbes, and long-term exposure could all feel effects if waste disposal goes unchecked. We’ve seen “harmless” compounds from years ago—think PCBs or certain solvents—get new toxic ratings after more thorough research.
For anyone storing, moving, or experimenting with chemicals like this, a little care pays off. Regular training, sharing up-to-date safety sheets, and keeping emergency plans sharp all matter. If something looks cheap or quick but skips safety, scrap it. People have power to ask questions, demand transparency from suppliers, or call for better standards. That doesn’t just protect workers; it helps communities living near warehouses, dumps, or labs breathe easier.
3,4-Dimethoxy thiophene doesn’t seem to spell disaster, but nobody loses by giving chemicals the respect they deserve. It’s possible to innovate and protect both people and the planet, if vigilance stays strong.
3,4-Dimethoxy thiophene looks harmless at first. Colorless claws, a hint of that sweet ether smell, and suddenly—if you turn your back—it can go from a valuable reagent to a risky mess. Anyone who’s worked in a lab knows how quickly things go sideways after a single oversight with solvents or thiophenes. One missed cap or a forgotten fridge shelf can be all it takes for a bottle to react or degrade, rendering it useless—sometimes even dangerous.
Direct sunlight and room heat play a bigger role here than most realize. Every chemist can remember a bottle or two that darkened over the weeks, no matter how clean it looked. 3,4-Dimethoxy thiophene holds up best in a cool, dry place, far from big swings in temperature. Shoving it next to a window or keeping it close to a radiator shortens its lifespan. At the bench, a dedicated chemical refrigerator—sturdy and not shared with sandwiches—gives this compound years instead of weeks. The bottle asks for zero direct sun, low moisture, and a climate that won’t bounce up and down.
Stories from the lab always include a mention of a strange-smelling bottle or a stuck cap. Oxygen won’t just degrade 3,4-dimethoxy thiophene over time; it changes the entire game. Gummed-up rings, surprising color changes, and tricky clean-up jobs usually come back to a little too much air creeping in. Reseal tight every time, use glass over plastic, and never use a leaky parafilm as your main lid. Argon or nitrogen gas, if available, gives extra peace of mind for longer storage.
Contaminants are more common than people want to admit. Tap water, oily hands, or just plain dust can get inside during an innocent rush. Even small traces of acid or base from glassware linger longer on freshly-washed containers than anyone wants to admit. The next use reveals an off smell or ruined product because the storage setup went sloppy. Always choose clean, dry glassware, wear gloves, and label the date every time you open and reseal the bottle. These basics build confidence and keep the material ready for research—not ruined before use.
Most labs run on a budget, so few get perfect conditions. A small fridge, a well-marked drawer, a reliable supply of argon—it’s not luxury, just good sense. Making habits around double-checking lids, running regular visual checks, and rotating stock shrink the risk of throwing out five hundred dollars’ worth of chemical. An open notebook by the storage area—listing bottle dates and recent uses—stops the “was this open last week?” game that’s cost labs real money and safety breaks.
Manual routines protect more than any automated system. The temptation to rush, borrow bottles, or skip the labeling step undoes months of careful storage. Teams that invest in honest checklists, clear labeling, and reminders tend to build a safety culture. This approach guards expensive chemicals and the people using them, while the fancy systems sometimes distract from what works every day—good habits and a little patience. No fancy fridge can replace awareness in the lab.
It’s easy to breeze past long-winded chemical names in research papers or pharmaceutical news, but 3,4-Dimethoxy thiophene actually plays a hands-on role in several growing industries. In the lab, this sulfur-containing ring caught my eye early on, since chemists seem to use it as a clever shortcut for building bigger and smarter molecules. I’ve seen it most in organic electronics, drug design, specialty polymers, and even agriculture.
On the electronics front, researchers could talk all day about the potential of thiophene derivatives. In practical terms, I’ve watched teams mix 3,4-Dimethoxy thiophene into conductive polymers. These polymers end up in flexible displays, sensors, and even solar cells. The methoxy groups attached to the ring actually help improve conductivity and stability—factors that matter when engineers try to scale up production. I remember one project where swapping out other monomers for this compound led to better charge transport in OLEDs. Devices got brighter and lived longer, which means fewer replacements and happier customers down the line.
Drug chemists love molecular scaffolds that offer both flexibility and a handle for further modifications. 3,4-Dimethoxy thiophene fits this description well. I’ve worked on syntheses involving this compound as a starting point for antifungal and anti-inflammatory drugs. Its structure gives medicinal chemists room to attach other chemical groups, nudging the final drug closer to the desired effect in the body. Large pharmaceutical companies sometimes keep their exact procedures confidential, but patent data shows that thiophene rings turn up in several new drug candidates for both cancer and infectious disease.
Besides electronics and pharma, materials science labs find uses for 3,4-Dimethoxy thiophene in the development of high-performance plastics. Its unique combination of sulfur and oxygen atoms changes how the polymer chains interact, making films tougher or more flexible, depending on what the engineer is after. In practical settings, companies push for lighter, more durable packaging materials or coatings that hold up against moisture and chemical stress. The custom-tailoring often starts with rare compounds like this one.
A less obvious application comes from the agrochemical world. Laboratories use 3,4-Dimethoxy thiophene as a scaffold in synthetic routes toward new fungicides and pesticides. The compound’s structure helps chemists design molecules that break down more safely in the environment or target specific pests. Agricultural policy circles debate safety profiles all the time, so researchers keep refining these molecules for better biodegradability and lower toxicity.
Supply-chain reliability often limits broader adoption of specialty compounds. As someone who’s watched project deadlines slip due to missing chemicals, I know first-hand how crucial it feels to secure a robust supplier network. Prices for rare precursors like this one might keep some smaller companies out of the game, so investment in scalable synthesis or green chemistry methods could open new doors. Collaboration between academic researchers, startups, and established manufacturers might give technicians and scientists the breathing room to turn creative lab ideas into real products—without sweating about backorders or regulatory bottlenecks.
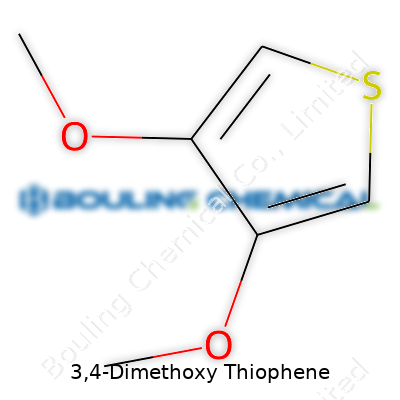

| Names | |
| Preferred IUPAC name | 3,4-Dimethoxythiophene |
| Other names |
3,4-Dimethoxythiophene Thiophene, 3,4-dimethoxy- 3,4-Bis(methoxy)thiophene |
| Pronunciation | /ˌθaɪ.oʊˈfiːn/ |
| Identifiers | |
| CAS Number | 10238-21-8 |
| 3D model (JSmol) | `3Dmol__Mol3D__pdb:MFCD00143352` |
| Beilstein Reference | 143880 |
| ChEBI | CHEBI:141107 |
| ChEMBL | CHEMBL3184858 |
| ChemSpider | 21580938 |
| DrugBank | DB08358 |
| ECHA InfoCard | 03c866a2-f035-4434-a121-0be6c8c4586e |
| EC Number | 61092-07-3 |
| Gmelin Reference | 8928 |
| KEGG | C16673 |
| MeSH | D016708 |
| PubChem CID | 1208194 |
| RTECS number | KL8575000 |
| UNII | MP1Q7W5F2J |
| UN number | NA |
| CompTox Dashboard (EPA) | DTXSID4044359 |
| Properties | |
| Chemical formula | C6H8O2S |
| Molar mass | 186.24 g/mol |
| Appearance | Light yellow to yellow liquid |
| Odor | sweet |
| Density | 1.211 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 1.68 |
| Vapor pressure | 1.7 hPa (25 °C) |
| Acidity (pKa) | 7.06 |
| Basicity (pKb) | 14.38 |
| Magnetic susceptibility (χ) | -37.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.568 |
| Viscosity | Liquid |
| Dipole moment | 1.85 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 322.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1552 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302+H312+H332-Harmful if swallowed, in contact with skin or if inhaled. |
| Precautionary statements | P261, P264, P271, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P332+P313, P337+P313, P362+P364 |
| Flash point | Flash point: 113°C |
| Lethal dose or concentration | LD50 (oral, rat): > 2000 mg/kg |
| NIOSH | SN8330000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10g |
| Related compounds | |
| Related compounds |
3,4-Ethylenedioxythiophene Thiophene 2,5-Dimethoxytetrahydrofuran 2,5-Dimethoxytoluene |